A look at the new FITC- and APC-conjugated rabbit IgG isotype control antibodies and why you should use them in your research.
You can now get a rabbit IgG isotype control antibody that’s conjugated to FITC, APC or PE. This has been in development for a while now as we’ve had a lot of requests for conjugated isotype controls. A conjugated isotype control like this is ideal to use alongside the unique conjugated rabbit primary antibodies that target specific extracellular proteins that you probably already come to us for.
These isotype controls are optimized for staining cell surface protein and so they’re perfect for flow cytometry.
You’ll find them in the catalog as Rabbit IgG Isotype Control-FITC (#RIC-001-F), Rabbit IgG Isotype Control-APC (#RIC-001-APC) and Rabbit IgG Isotype Control-PE (#RIC-001-PE), and you’ll notice a few things about them:
- Unlike some vendors, the exact concentration is clearly listed, as is the formulation it’s supplied in (PBS, pH 7.4, 1% BSA, 0.05% NaN3)
- They don’t include any glycerol
- They’re supported by robust testing and come with clear flow cytometry validation data
- The conjugation protocol we use is the same as our other antibodies, so the fluorophore to protein ratio (F:P ratio) is the same as theirs (5 to 10 fluorophore molecules per antibody molecules) – you can clearly see the dye when it’s in solution
Expanding Your Control Options
There’s more in the pipeline. Right now, you can get FITC-, APC- and PE-conjugated isotype control, but isotype controls should be used for a whole range of immunoassays. So, we’re going to create more options. Keep your eye on the catalog because soon we’ll have a few more isotype control antibodies to choose from, such as
- Unconjugated
- Carrier-free (no BSA or NaN3)
- Conjugated to other fluorophores, like an ATTO-Fluor
What is an Isotype Control?
An isotype control is an antibody that looks like your regular antibody (it’s the same immunoglobulin isotype – or at least it should be!), but it doesn’t bind to a specific target like you want your usual primary antibody to.
Why use an Isotype Control?
The reason’s in the name: it’s a control. It’s a negative control that lets your account for background signal in your sample. Background can arise for several reasons, but one is Fc receptor interactions. You see, while your focus is usually on the Fab region, where the useful target-specific binding happens, the Fc region can also be bound. This is because you’ll find Fc receptors on a range of cells, proteins, and lipids that you have no interest in, and these can bind your antibody, causing background signal.
By running a control experiment alongside your main experiment, using the isotype control instead of your usual primary antibody, and ensuring all other conditions are the same – concentration, buffers, temperature – you can determine the extent of non-specific background (i.e., establish your threshold for unstained samples). In short, you can pick out signal from noise much more easily.
For flow cytometry, you might already run a ‘fluorescence minus one’ (FMO) control where you include everything as usual except the reagent you’re establishing your threshold for. Isotype controls are a great alternative as long as the isotype control has the same nonspecific binding capacity as your experimental antibody.
Developing the FITC-Conjugated Isotype Control
It’s important that a control reagent like this is as close to the experimental antibody you’re using as possible. So, we label these new isotype control using the same protocols that we developed and refined for labeling our regular conjugated antibodies. This means the isotype control has the same fluorophore to protein ratio (F:P ratio) as any other conjugated antibody you get from us (5–10 fluorophore molecules per antibody molecule). These isotype controls also undergo the same purification process after labeling so everything is at the same standard.
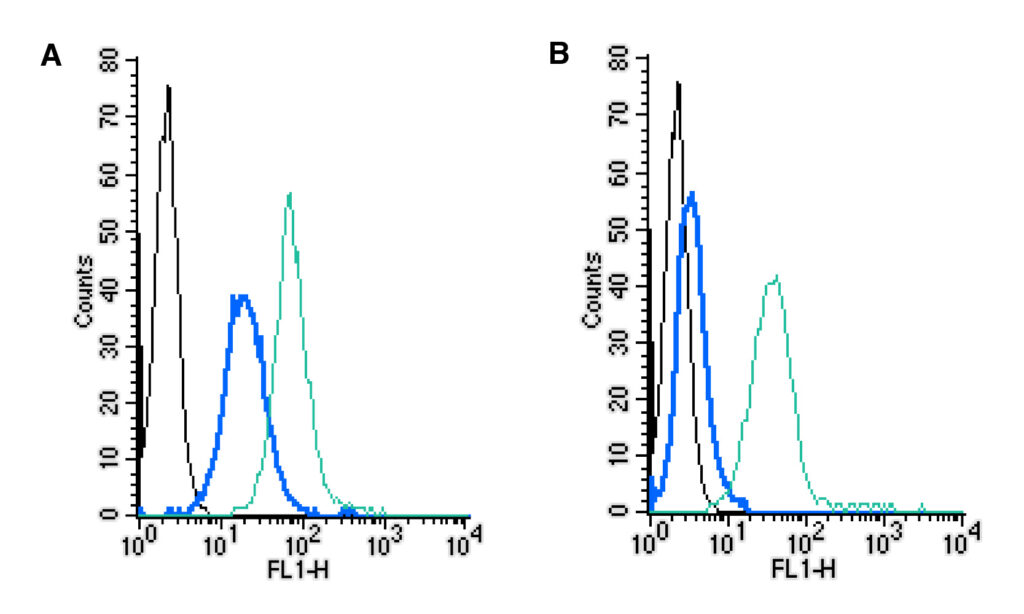
Once we have a working reagent, we test the isotype control in tandem with several experimental antibodies in multiple cell types (J774, BV-2, Jurkat, THP-1, and TK-1) and species (mouse and human). And since flow cytometry is the likely focus for these reagents, we validated by staining cell surface proteins on living cells and analyzing this via flow cytometry. As we expand the range, we’ll validate new isotype controls in a range of immunoassays.
The end result? A robust control to add to your flow cytometry work, made and tested in-house, to give you more confidence in your data.
