Venom proteins and peptides have emerged as invaluable tools for research, drug discovery and drug development due to their small size, rigid structure, high potency and selectivity. The use of labeled toxins will open up new research avenues which will help to better understand ion channel function, with regard to biophysical and pharmacological properties, tissue distribution, and to isolate specific channel activities in living cells.
Introduction
Venom proteins and peptides have emerged as invaluable tools for research, drug discovery and drug development.1,2 Ion channel research tools that are currently available include highly specific antibodies and radiolabeled toxins. Although Alomone Labs offers several antibodies with extracellular epitopes, most work with “live-cells” or native tissues is hampered by the need to permeabilize the cells (for intracellular epitopes) or by the high sequence homology between ion channel extracellular domains, which makes it difficult to isolate specific epitopes. Using radiolabeled toxins requires working with radioactivity, which limits the laboratories that can use such techniques and another drawback is that the resolution obtained by autoradiography is low.3 The ability to produce toxins labeled with a detectable probe (radioligands, biotin or fluorescent dyes) has radically broadened the use of toxins to include determination of ion channel extracellular localization, distribution, affinities, and number of binding sites, in live cells. Detectable probes can be introduced into the toxins by a number of techniques:
a) chemical synthesis at the terminal ends, or at a specified location4,5
b) site directed mutagenesis to liable and active amino acid residues6-11
c) use of specific chemistries including N-hydroxysuccinimidyl ester chemistry (for ε-amino groups of lysine), maleimide chemistry (for sulphidryl moieties) and iodonization of tyrosines.12-21
The last group of methods, although the most common, are crude, and the detectable probe can bind to the protein at unknown locations and with unknown stochiometry. Even more importantly, it may bind to crucial residues which participate in the toxin-receptor binding interface. Numerous papers have described the structure/function of toxins, based on mutagenesis experiments, molecular simulation, and structure determination. The mode of action of these toxins can be roughly divided into two major groups: the “pore-plungers” and the “voltage-sensor modulators” or “gating-modifiers”. “Pore-plungers” bind to the pore region and physically block the ion transport through the pore. For example, the majority of K+ channel blockers are characterized by the presence of a “dyad” or “triad”, having a Lys in structural proximity to either one or two hydrophobic residues (Phe, Tyr, Trp) (Fig. 1).21 The Lys residue is “plunged” into the pore and interacts with the channel’s Asp residues inside the pore, while the hydrophobic residues interact with hydrophobic residues on the pore outer-surface.22,23 If the exposed Lys is bound to a hindering dye moiety or the Tyr residue is iodinated, the labeled protein may be less active than the native form. This can also occur with any bulky moiety at the toxin-channel interface, or with a secondary structural perturbation due to dye or linker conjugation. In light of these facts, dyes or radioligands should be selectively introduced on the other “face” (or “back”) of the toxin, at a specific location, with well defined stochiometry. Site-specific introduction of fluorescent dyes was demonstrated for a number of toxins, including Iberiotoxin (#STI-400), Hongotoxin (#STH-400), and ShK (#STS-400) K+-channels blockers.3-5,10,11 Hafidi et al. were able to stain and localize KCa1.1 (BK or Slo K+ channels) in cochlear hair cells and tissues, using a mutated Asp19Cys-Iberiotoxin labeled with Alexa488.10 Bingham et al. chemically introduced a biotin derivative of Lys at the same Asp19 of Iberiotoxin, and were able to visualize KCa1.1 stably-transfected HEK-293 cells using Streptavidin–Alexa488.4
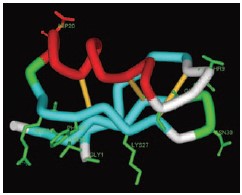
NMR structure of Agitoxin-2 (1AGT.pdb). Gly1, Thr9, Gly10, Ser11, Arg24, Phe25, Lys27, Met29, Asn30 and Thr36 have been found to participate in the interaction with KV1.3 (marked in green)26. Asp20, located at the opposite face of the protein was replaced with Cys (marked in red, see article).
Images were generated using the ViewerLite freeware (Accelrys Inc.).
Hongotoxin is a known blocker for KV1.1, KV1.2, KV1.3 and KV1.6, with IC50 of 31pM, 170 pM, 86 pM and 6nM respectively.(see review in this Modulator issue) By introducing Cys19Ala of Hongotoxin (Ala19Cys-Hongotoxin, the structural equivalent to Asp19 of Iberiotoxin) and binding to a number of fluorescent dyes, Pragl et al. were able to obtain a toxin with the same activity as the native toxin, which binds to cells and colocalizes with KV1.2 in brain slices.3 Furthermore, Freudenthaler et al. were able to visualize single channels on Jurkat T-cell lymphocytes using ultrasensitive microscopic methods to determine the dissociation and off-rates of Hongotoxin, using the Cy5-Ala19Cys-Hongotoxin.11 In another example, ShK, a toxin isolated from the sea anemone Stichodactyla helianthus, was found to be a highly potent KV1.3 blocker with IC50 of 11pM.27 Beeton et al. have introduced a number of fluorescent and biotin tags to the N-terminal arginine residue, and used these toxins to discriminate between activated T-lymphocytes and resting T-lymphocytes, which are differentiated by the high expression levels of voltage-gated KV1.3 channels.5 Furthermore, the authors reported detection at a level as low as 600 channels per cell.5
Agitoxin-2, a 38 amino acid toxin containing 3 disulfide bonds, isolated from the venom of the scorpion Leiurus quinquestriatus hebraeus, was successfully expressed and its NMR structure solved (1AGT.pdb).24-25 Biochemical, pharmacological and molecular simulation experiments have identified the residues Gly1, Thr9, Gly10, Ser11, Arg24, Phe25, Lys 27, Met 29, Asn30 and Thr36 at the agitoxin-2/KV-channel interface (Fig. 1).26 As was outlined previously, point mutations were introduced at the other non-interacting “face” of the toxin, thus reducing the probability of hampering the toxin activity after introduction of a detectable probe. Asp20 of Agitoxin-2 (the structural homolog of Asp19 Iberiotoxin and Ala19 Hongotoxin) was replaced by Cys, and this mutant was recombinantly expressed, purified, and reacted with TAMRA-maleimide, to form Asp20Cys-AgiTx-2-TAMRA Agitoxin-2-Cys-TAMRA (#RTA-420-T). Agitoxin-2-Cys-TAMRA was bioassayed for the inhibition of KV1.3 currents, both in Xenopus oocytes and KV1.3 stably-transfected HEK cells, and it activity was found to be identical to that of Agitoxin-2 (#STA-420) (Fig. 4). Furthermore, Agitoxin-2-Cys-TAMRA was tested for its ability to fluorescently-label KV1.3 stably-transfected HEK cells. As seen in Fig. 2 and 3, Agitoxin-2-Cys-TAMRA was able to detect the functional KV1.3 channels in live cells without fixation, and labeled the channels on the cell membranes, as seen by the overlay picture, in less than 30 minutes. Moreover, no labeling was observed when the cells were pre-incubated with a competing non-labeled Agitoxin-2, and no background binding was observed on non-transfected HEK cells (Fig. 2 and 3).
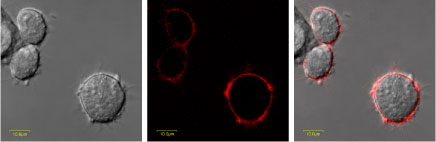
Confocal image of KV1.3 expressing HEK-293 cells labeled with a fluorescent Agitoxin-2-Cys-TAMRA (#RTA-420-T).KV1.3 expressing HEK-293 cells were grown on glass cover-slips, incubated in the presence of 50nM Agitoxin-2-Cys-TAMRA for 30 minutes at room temperature and washed with PBS.Left: Bright light field image of the cells.
Middle: Image acquired with a Rodamine filter.
Right: Merge of both images. As can be seen, Agitoxin-2-Cys-TAMRA is localized with the membrane-anchored KV1.3 K+ channel.We would like to thank Mr. Vladimir Kiss and Dr. Noga Alagem from the Weizmann Institute of Science for their help with the confocal microscope images.
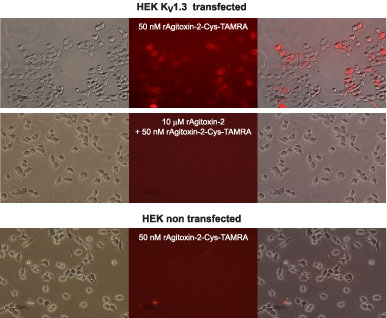
Top: Staining of HEK-293 cells stably expressing KV1.3 channels with 50nM of Agitoxin-2-Cys-TAMRA (#RTA-420-T). Cells were incubated for 30 minutes with the fluorescent toxin, washed with PBS and visualized. In the middle section the image acquired with a Rodamine filter is shown and on the right it is merged with the bright light image of the same field to illustrate cellular staining.
Middle: Displacement of labeled toxin by Agitoxin-2 (#STA-420). The same preparation as the one shown on top was pre-incubated for 30 minutes with 10μM of Agitoxin-2, followed by another 30 minute incubation after addition of 50nM Agitoxin-2-Cys-TAMRA.Bottom: Agitoxin-2-Cys-TAMRA does not stain non-transfected HEK-293 cells. The procedure was as in the top panel.
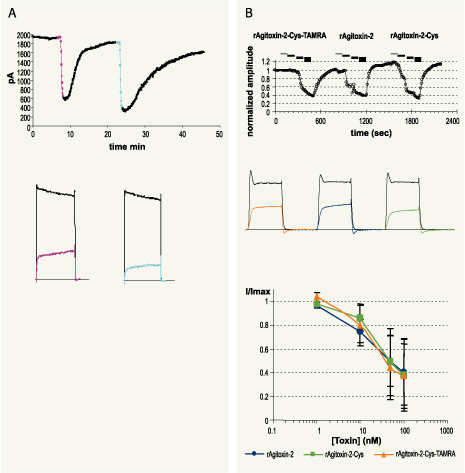
A. KV current inhibition by Agitoxin-2 (#STA-420) (100nM, pink) and Agitoxin-2-Cys-TAMRA (#RTA-420-T) (100nM, blue) in KV1.3 transfected HEK-293 cells.
Top: Time course of KV1.3 current amplitude is presented. Currents were elicited from holding potential of -100 mV by 200 ms voltage pulses to +50 mV, every 10 seconds. Periods of toxin perfusions are indicated according to the color code defined above.
Bottom: Superimposed traces taken from the same experiment shown on top, before (black) or during toxin perfusion (pink or blue).B. Dose response of KV1.3 current inhibition in Xenopus oocytes for Agitoxin-2, Agitoxin-2-Cys-TAMRA and Agitoxin-2-Cys (product has been discontinued).Top: Time course of current amplitude, upon toxin perfusion. Currents were elicited from resting potential of -100 mV, by a 100 ms test pulse to 0 mV, delivered every 10 seconds. The bars above the plot represent periods of toxin perfusion, for each toxin sequential applications of the following concentrations: 1, 10, 50 and 100 nM, where the toxin species is indicated.
Middle: Superimposed traces representing currents under control conditions (black) and during perfusion of 100 nM toxin: Agitoxin-2-Cys-TAMRA (orange), Agitoxin-2 (blue) and Agitoxin-2-Cys (green).Bottom: Dose reponse of toxins on current inhibition (n=3 for each toxin, mean and SD). See inset for color coding.
Alomone Labs is proud to launch Agitoxin-2- Cys-TAMRA as the first member in a new line of fluorescently-labeled toxins, which we believe will be invaluable tools for channel research both in live cells and in tissues. Please check our website for new labeled toxins.
References
- Bogin, O. (2005) Venom Peptides and their Mimetics as Potential Drugs, Modulator Issue #19.
- Lewis, R.J. and Garcia M.L. (2003) Nat. Rev. Drug. Discov. 2, 790.
- Pragl, B. et al. (2003) Bioconjug. Chem. 13, 416.
- Bingham, J.P. et al. (2006) Bioconjug. Chem. 17, 689.
- Beeton, C. et al. (2003) J. Biol. Chem. 278, 9928.
- Wanner, S.G. et al. (1999) Biochemistry 38, 5392.
- Koschak, A. et al. (1997) Biochemistry 36, 1943.
- Grunnet. M. et al. (1999) Am. J. Physiol. 277, G22.
- Shimony, E. et al. (1994) Protein Eng. 7, 503.
- Hafidi, A. et al. (2005) Neuroscience 130, 475.
- Freudenthaler, G. et al. (2002) Histochem. Cell Biol. 117, 197.
- Darbon, H. and Angelides, K.J. (1984) J. Biol. Chem. 259, 6074.
- Angelides, K.J. (1989) Methods Cell Biol. 29, 29.
- Jones, O. T. et al. (1989) Science 244, 1189.
- Cohen, M.W. et al. (1991) J. Neurosci. 11, 1032.
- Angelo, K. et al. (2003) Pflugers Arch. 447, 55.
- Massensini, A.R. et al. (2002) J. Neurosci. Methods 116, 189.
- Sekine-Aizawa, Y. and Huganir, R.L. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 17114.
- Wang, H. et al. (2003) Nature 421, 384.
- Racape, J. et al. (2002) J. Biol. Chem. 277, 3886.
- Jimenez-Gonzalez, C. et al. (2003) Eur. J. Neurosci. 18, 2175.
- Mouhat, S. et al. (2005) J. Pept. Sci. 11, 65.
- Rodrıguez de la Vega, R.C. et al. (2003) Trends Pharmacol. Sci. 24, 222.
- Garcia, M.L. et al. (1994) Biochemistry 33, 6834.
- Krezel, A.M. et al. (1995) Protein Sci. 4, 1478.
- Gao, Y.D. and Garcia, M.L (2003) Proteins 52, 146.