Transient (“A-Type”), voltage-dependent K+ currents play important roles in both neurons, cardiac myocytes and smooth muscles. The KV4 channels subfamily were found to interact with a number of ancillary subunit proteins which modulate their activity, conferring their “A-Type” characteristics to the resulting currents.
Novel Peptidyl Toxin Blockers
The KV4 subfamily of voltage dependent K+ channels is the mammalian parallel of the Drosophila Shal K+channel. These currents have “A-type” namely transient profile, which is characterized by fast activation followed by fast inactivation, upon depolarization of the membrane potential. Both the KV1 and the KV4 families generate “A-type” currents, with the latter activating at lower voltage1,2.
There are three members in this subfamily designated KV4.1-3. Alternative splicing of KV4.3 creates three variant products, further contributing to the divergence of the subfamily2. Members of the KV4 subfamily are expressed in heart3, neurons4 and some smooth muscle such as the uterus, where estrogen down regulates KV4.3 channels (which is correlated to their down regulation with pregnancy propagation)5. In the heart KV4.2 and KV4.3 channels underlie part of Ito, with differential expression in different parts of the heart and across species3. In neurons KV4 channels are correlated with the sub-threshold transient K+ currents4. In the substantia nigra, dopaminargic neurons express KV4.3, which play a key role in the control of an intrinsic pacemaker activity6.
KV4 channels were shown to interact with auxiliary β subunits (KVβ1 and 2), via a C-terminal interaction, which increase their membrane expression leading to larger current density7. Interestingly, KVβ1.2 confers O2 sensitivity to KV4.2 channels, which suggests a role in response to hypoxia8.
Recently, many reports show that KV4 channels interact with, and their properties are modulated by, K+Channel Interacting Proteins (KChIP), such as CALP and DREAM. In general, KChIP increases membrane expression, slows the inactivation, affects intracellular trafficking and enhances recovery from inactivation of KV4 channels6,9-11,18,19. In a recent work by Kim et al., the 3D structures of KV4.2 – K ChiP2 was resolved by electron microscopy at 21 resolution20. KV4.2 channels were also shown to interact with PSD9512 and frequenin13.
Phosphorylation also plays a major role in KV4 channel function and modulation as the KV4.2 sequence contains consensus sites for ERK/MAPK, PKA, PKC and CaMKII14. These channels were shown to be phosphorylated by PKA15. Both KV4.2 and KV4.3 are specifically and reversibly blocked by the peptidyl toxins, Phrixotoxin-216 and Heteropodatoxin-217. Phrixotoxin-2 is isolated from Phrixotrichus auratus tarantula spider venom while rHeteropodatoxin-2 is an E.coli expressed recombinant toxin, originally isolated from the venom of the Heteropoda venatoria spider. Phrixotoxin-2 blocked KV4.2 and KV4.3 channels in COS-transfected cells with an IC50 of 34 and 71 nM respectively16. In Xenopus oocytes the toxin was less potent and inhibited KV4.3 with an IC50 of 650nM. Heteropodatoxin-2 blocked transient outward K+ current (Ito) in ventricular myocytes in a voltage dependent manner with an IC50 (at –10 mV) of 16 nM17.
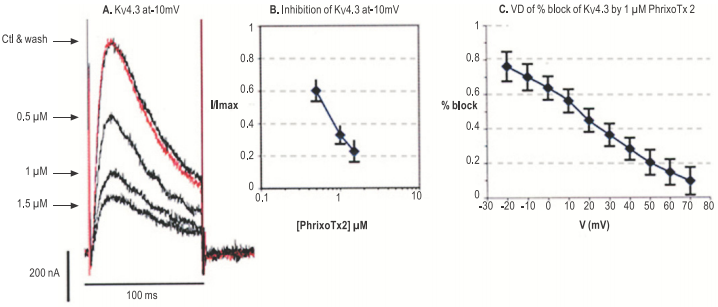
A. Traces of current response to 100 ms depolarization from holding potential of –100 mV to -10 mV, applied every 10s. before (red), during and after wash of the indicated Phrixotoxin-2 (#STP-710) concentration. B. Mean and S.D. dose-response curve for 3 experiments as in A. C. Mean and S.D. voltage dependence of the percentage inhibition caused by 1 µM phrixotoxin 2 (n=3).
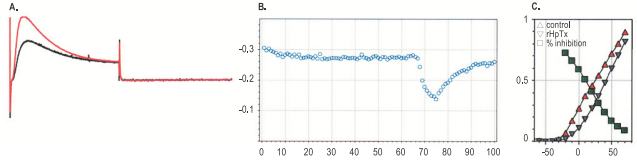
A. Current responses to 100 ms depolarization to 0 mV from holding potential of -100 mV, delivered every 10 seconds before (red) and during application of 500 nM Heteropodatoxin-2 (#STH-340). B. Time course of amplitude inhibition. The horizontal bars indicate the periods of Heteropodatoxin-2 perfusion. C. I-V relationships for KV4.2 before and during Heteropodatoxin-2 perfusion. Also included the fraction of inhibited current as function of the voltage step (green squares).
References
- Hille, B. (2001) Ion Channels of excitable membranes. 3rd edition.
- Coetzee, W. A. et al. (1999) Anals N. Y. Acad. Sci. 868, 233.
- Brahmajothi, M. V. et al. (1999) J. Gen. Physiol. 113, 581.
- Seodio, P. and Rudy, B. (1998) J. Neurophysiol. 79, 1081.
- Song, M. et al. (2001) J. Biol. Chem. 276, 31883.
- Liss, B. et al. (2001) EMBO J. 20, 5715.
- Yang, E. K. et al. (2001) J. Biol. Chem. 276, 4839.
- Perez-Garcia, M. T. et al. (1999) J. Gen. Physiol. 113, 897.
- Bahring, R. et al. (2001) J. Biol. Chem. 276, 23888.
- Holmqvist, M. H. et al. (2002) P.N.A.S. USA 99, 1035.
- Morohashi, Y. et al. (2002) J. Biol. Chem. 277, 14965.
- Wong, W. et al. (2002) J. Biol. Chem. 277, 20423.
- Nakamura, T. Y. et al. (2001) P.N.A.S. USA 98, 12808.
- Varga, A. W. et al. (2000) Learn. Mem. 7, 321.
- Anderson, A. E. et al. (2000) J. Biol. Chem. 275, 5337.
- Diochot, S. et al. (1999) Br. J. of Pharmacol. 126, 251.
- Sanguinetti, M.C. et al. (1997) Mol. Pharmacol. 51, 491.
- Shibata, R. et al. (2003) J. Biol. Chem. 278, 36445.
- Patel, S.P. et al. (2004) J. Physiol. (Epub ahead of print).
- Kim, L.A. (2004) Neuron. 41, 513.