Nerve Growth Factor (NGF) is one of the founding and best-characterized members of the neurotrophin family. This family comprises a unique collection of polypeptidic growth factors that promote the proliferation, differentiation, survival and death of neuronal and non-neuronal cells. Alomone Labs offers an extensive list of neurotrophin proteins and related antibodies. These valuable research tools continue their contribution in the ongoing research regarding the downstream mechanisms induced by NGF signaling, as described and presented below.
Introduction
NGF was discovered 50 years ago40 and since its elucidation, its role in neural development has been extensively characterized. However, recent findings point to unexpected actions of NGF on non-neuronal cells and suggest that developmental effects are only one aspect of the biology of NGF. In the central nervous system (CNS), in addition to its established functions in neuronal cell survival, NGF also mediates additional higher-order activities, such as learning, memory and behavior. Alterations in NGF levels are implicated in neurodegenerative disorders, such as Alzheimer’s disease (AD) and Huntington’s disease, as well as psychiatric disorders, including depression and substance abuse12.
NGF mediates its signal through two distinct classes of cell surface receptors: the shared p75 neurotrophin receptor (p75NTR) and the Trk receptor. NGF binds most specifically to TrkA receptor. While p75NTR can bind to each of the neurotrophin family members, it also regulates the binding affinity of Trk receptors to their cognate ligands12. NGF sends its survival signals through the activation of TrkA and can induce cell death by binding to p75NTR 63.
NGF Used as a Maintenance and Differentiating Agent in Tissue Cultures in vitro
NGF is required for the development of sympathetic neurons and a subset of sensory neurons. Our current knowledge on the molecular mechanisms underlying the biological functions of NGF is in part based on the studies with rat pheochromocytoma (PC12) cells, which differentiate into sympathetic neuron-like cells upon NGF treatment30.
Native mouse NGF 2.5S protein (99%) (#N-240) and Native mouse NGF 2.5S protein (>95%) (#N-100) were used in countless of works in order to maintain and differentiate a variety of culture cell types in vitro: PC12 cells10,14,16,25,45,50,61, various primary neuronal cultures4,15,34-35,38,47,54 and other types of cultures, such as adrenal cell cultures55. Native mouse NGF 7S protein (#N-130) was also used as a key maintenance factor in numerous primary neuronal cultures7,20,23,27,36-37,48,52-53,64 and PC12 cells62 as well.
Selected works mention the general addition of NGF as a fundamental neurotrophic factor for differentiating various tissue cultures: PC121,5,13,17,49,56-57,65 as well as primary neuronal cultures3,6,11,21-22,24,26,28-29,31-33,42-43,51,58-59. Figure 1 illustrates long term culturing of motor cortex slices in the presence of NGF and other neurotrophins59, while Figure 2 illustrates long term culturing of PC12 in the presence of NGF46.
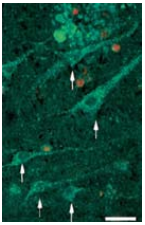
Viability staining of a motor cortex slice (layer III) kept in basal medium (R16) supplemented with an Alomone Labs NGF product, Recombinant human BDNF protein (#B-250), and Recombinant human Neurotrophin-3 (NT-3) protein (#N-260) for 48 days. Arrows indicate viable neurons that have retained both esterase activity (green cytoplasm) and intact membranes (without red nuclei). Scale bar, 35 μm.Adapted from reference 59 with permission of Federation of American Societies for Experimental Biology (FASEB).
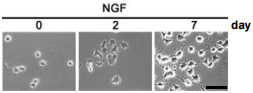
Stable clones of PC12 cells transfected with mock (pEGFP-N1) constructs were subjected to an Alomone Labs NGF product (50 ng/ml) treatment. Cells were observed under the microscope with phase-contrast after 0, 2, or 7 days of NGF treatment. Scale bar, 50 µm.
Adapted from reference 46 with permission of The American Society for Biochemistry and Molecular Biology.
The Effect of NGF on Neurite Outgrowth
Poly ADP-ribosylation is a transient posttranslational modification of proteins, mainly catalyzed by poly (ADP-ribose) polymerase-1 (PARP-1). This highly conserved nuclear protein is activated rapidly in response to DNA nick formation and promotes a fast DNA repair mechanism. The possible association between poly ADP-ribosylation and the activity of NGF (in addition to other neurotrophins), vis-à-vis life-or-death decisions in mammalian neurons was examined in a recent study60. PARP-1 was activated in rat cerebral cortical neurons briefly exposed to Native mouse NGF 2.5S protein (99%) or Native mouse NGF 2.5S protein (>95%) suggesting an alternative mode for PARP-1 activation in the absence of DNA damage. In addition, poly ADP-ribosylation was involved in the neurotrophic activity of NGF-induced neurite outgrowth in differentiating PC12 cells60 (Figure 3). A fast loosening of the highly condensed chromatin structure by poly ADP-ribosylation of histone H1, which renders DNA accessible to transcription and repair, may underlie the role of poly ADP-ribosylation in neurotrophic activity60.
During the development of the amniote peripheral nervous system, the initial trajectory of primary sensory axons is determined largely by the action of axon repellents. Although there is evidence suggesting that SEMA3A contributes to the repellent activity of the dermamyotome, the nature of the activity secreted by the notochord remains to be determined. Using a spectrum of different axon populations to assay the notochord activity, it was demonstrated that SEMA3A probably contributes to notochord-mediated repulsion. Results indicate that stage 36 DRG axons, cultured in the presence of an Alomone Labs NGF product, are repelled by stage 17-21 notochords2 (Figure 4). Moreover, retinal axons, which are insensitive to SEMA3A, are also repelled by the notochord. The results lead to the conclusion that multiple factors act in concert to guide axons in this system, and that further notochord repellents remain to be identified2.
During differentiation, neurons increase phospholipid biosynthesis to provide new membrane for neurite growth. The study presented, investigated the regulation of phosphatidylcholine (PC) biosynthesis during differentiation in PC12 cells. During neurite outgrowth, NGF doubled the amount of cellular PC and CTP: phosphocholine cytidylyltransferase (CT) activity. CTβ2 mRNA increased within 1 day of Native mouse NGF 2.5S protein (>95%) application, prior to the formation of visible neurites, and continued to increase during neurite growth. When neurites retracted in response to NGF withdrawal, CTβ2 mRNA, protein, and CT activity decreased. NGF specifically activated CTβ2 by promoting its translocation from the cytosol to membranes. In contrast, NGF did not alter neither CTα expression nor its translocation. Together, these data suggest that the CTβ2 isoform is specifically up-regulated and activated during neuronal differentiation to increase PC biosynthesis for growing neurites10.
Another work demonstrated that the expression of leukemia inhibitory factor receptor (LIFR), one of the signaling molecules shared by several neuropoietic cytokines of the interleukin-6 family, is specifically up-regulated in PC12 cells following treatment with an Alomone Labs NGF product46. Attenuation of LIFR signaling through stable transfection of antisense (AS) or dominant negative (DN) LIFR constructs enhanced NGF-induced neurite extension in PC12 cells (Figure 5). The findings in this study demonstrate that LIFR expression can be specifically induced by NGF and, apart from its known function in cell survival and phenotype development, activated LIFR signaling can exert negative regulatory effects on neurite extension and branching of sympathetic neurons46.

A) Neurite outgrowth in PC12 cells after 48 h incubation with Native mouse NGF 2.5S protein (99%) (#N-240) or Native mouse NGF 2.5S protein (>95%) (#N-100) (50 ng/ml) in the presence or absence of PARP-1 inhibitor (3-AB; 1 mM). Confocal microscope images are presented, showing morphological changes and fluorescent immunolabeling of MAP2 in fixed cells (upper panels). Cells were also visualized by phase contrast in transmitted light (n=3), (lower panels). Neurite outgrowth induced was prevented by the PARP-1 inhibitor 3-AB (0.5 mM). B) PARP-1 was activated in PC12 cells exposed for 5 min, 1 h, or 24 h to mouse NGF (same as in A). Poly ADP-ribosylated proteins were immunolabeled with anti-PAR. Poly ADP-ribosylation was suppressed in the presence of 6(5H)-phenanthridinone (Phen.), (25 μM). Confocal images indicate poly ADP-ribosylated proteins in the neuronal nuclei labeled with fluorescein-conjugated secondary antibody (n=3).
Adapted from reference 60 with permission of The Society for Neuroscience.
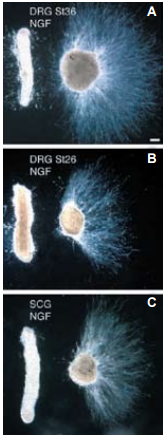
A)-C) Dark-field photomicrographs of representative co-cultures. A) Stage 36 (St36) DRG explants (n=90); B) Stage 26-27 (St26) DRG explants (n=55); C) Stage 36 SCG explants (n=28). Explants were cultured with an Alomone Labs NGF product. Scale bar, ~100 µm.
Adapted from reference 2 with permission of The Company of Biologists.
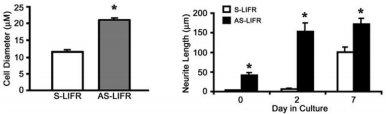
Quantification of the longest diameter (left) and length of the longest neurite (right) following treatment with an Alomone Labs NGF product was determined for S-LIFR and AS-LIFR cells.
*P < 0.01. The values represent the mean ± S.E., n=3 dishes.
Adapted from reference 46 with permission of The American Society for Biochemistry and Molecular Biology.
NGF as a Key Signaling Factor in the Nervous System
in vivo Use of NGF in Whole Animal Models of Neurodegenerative States
The link between NGF and Alzheimer’s disease (AD) was made in the 1980s based on studies of aged animals in which cholinergic neurons in the basal forebrain could be rescued with intra cerebroventricular injection of NGF. NGF delivery to the brain of patients appears to be an emerging potential therapeutic approach to treat neurodegenerative diseases, such as AD. The intranasal route of administration could provide an alternative to intra cerebroventricular infusion and gene therapy.
Knockout of NGF activity generates in transgenic anti NGF mice (AD11 mice) a progressive neurodegenerative phenotype resembling that of AD. Research done with this model examined whether and how the progressive neurodegenerative phenotype of AD11 mice could be prevented or ameliorated by pharmacological intranasal treatments with recombinant human NGF, Recombinant human beta-NGF protein (#N-245), at a relatively early phase of Alzheimer’s disease-like neurodegeneration. They demonstrate that the neurodegeneration induced by the expression of anti-NGF antibodies in AD11 mice can be largely reversed by NGF delivery through the olfactory route9 (Figure 6). It is assumed that the Alzheimer-like neurodegeneration in AD11 mice is linked to progressive behavioral deficits (visual recognition and spatial memories) starting from 4 months of age. Based on this assumption a study was initiated, to see whether intranasal administration of either Native mouse NGF 2.5S protein (99%) or Native mouse NGF 2.5S protein (>95%), started after the appearance of the first memory deficits, could revert the cognitive deficits in AD11 mice. Indeed, deficits exhibited by untreated AD11 mice could be rescued by the intranasal administration of NGF. Thus, this route of administration provides a promising way to deliver NGF to the brain from a therapeutic point of view19.
Uninjured C-type rat dorsal root ganglion (DRG) neurons predominantly express slowly inactivating TTX-resistant (TTX-R) and slowly repriming TTX-sensitive (TTX-S) Na+ currents. Following peripheral axotomy, TTX-R current density is reduced and rapidly repriming TTX-S currents emerge and predominate. Changes in Na+ current profile and kinetics in DRG neurons may substantially alter neuronal excitability and could contribute to some states of chronic pain associated with injury of sensory neurons. In the study presented, the authors investigated whether intrathecally administered NGF (product purchased from Alomone Labs) and Glial-Derived Neurotrophic Factor (GDNF), can ameliorate the axotomy-induced change in TTX-S Na+ current repriming kinetics. They show that this route of administration of NGF and GDNF, delivered individually, can partially reverse the effect of axotomy on the repriming kinetics of TTX-S Na+ currents. Their data indicate that both NGF and GDNF can partially reverse an important effect of axotomy on the electrogenic properties of sensory neurons and that their effect is additive39.
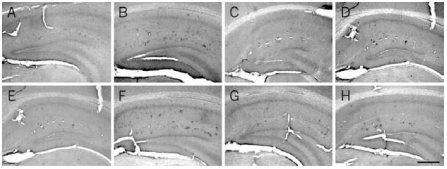
At 6 months of age, Aβ is localized in the soma of cells forming clusters in the hippocampus of AD11 mice (B) and is absent in control mice (A). At this age, treatment with Recombinant human beta-NGF protein (#N-245) (C) and Galantamine hydrobromide (GAL) (D) decreases the number of Aβ-positive clusters of cells. A more prolonged treatment, started at 4 months of age, further decreases the number of clusters in AD11-treated mice (G) compared with control mice (E) and untreated mice (F). H) Treatment with GAL does not result in a further decrease of the number of Aβ-positive clusters of cells. Scale bar, 100 μm.
Adapted from reference 9 with permission of The National Academy of Sciences of the USA (Copyright 2002).
Labeled NGF as a Key Research Tool in Axonal Transport in vitro
Axonal retrograde transport is essential for neuronal growth and survival. However, the nature and dynamics of the membrane compartments involved in this process are poorly characterized. In order to discover the mechanism of this pathway, several experimental systems were studied using Alomone Labs NGF in order to label it with biotin8 125I18,44 or Texas red37, for visualization and quantitative studies.
The axonal growth of cultured rat sympathetic neurons is inhibited when the ceramide content of distal axons, but not cell bodies, is increased. Using either Native mouse NGF 2.5S protein (99%) or Native mouse NGF 2.5S protein (>95%) radioiodinated, it was shown that treatment of distal axons with ceramide, inhibited the uptake of labeled-NGF by ~70%. Thus, the authors conclude that the inhibition of axonal growth by ceramide might be due, at least in part, to impaired endocytosis of NGF18.
Another work demonstrated using Native mouse NGF 2.5S protein (>95%) radiolabeled (125I-labeled NGF) and covalently cross-linked to beads, increased phosphorylation of TrkA and Akt, but not of mitogen-activated protein kinase in cultured rat sympathetic neurons. In addition, NGF beads or 125I-labeled NGF beads delivered to distal axons resulted in the survival of over 80% of the neurons for 30 hours, with little or no retrograde transport of 125I-labeled NGF. Application of free 125I-labeled produced 20-fold more retrograde transport, but only 29% of the neurons survived. Thus, in contrast to the widely accepted theory, a neuronal survival signal can reach the cell body unaccompanied by the NGF that initiated it44 (Figure 7).
Rapid internalization and retrograde trafficking of neurotrophin-Trk receptor complexes have been demonstrated in a number of systems and are thought to transmit trophic signals from terminals to neuronal cell bodies. In contrast, the internalization and trafficking of neurotrophin-p75NTR complexes are not well understood and were therefore investigated using either Native mouse NGF 2.5S protein (99%) or Native mouse NGF 2.5S protein (>95%) labeled with biotin8 (Figure 8).
Retrograde transport in living motor neurons was also visualized using the fluorescent fragment of tetanus toxin (TeNT HC) and Native mouse NGF 7S protein labeled with Texas red reagent. Importantly, TeNT HC and Texas Red labeled Native mouse NGF 7S protein, share the same retrograde transport organelles, which are characterized by the presence of p75NTR. The results of this study provide the first direct visualization of retrograde transport in living motor neurons, and reveal a novel retrograde route that could be used by both physiological ligands (i.e., neurotrophins) and TeNT to enter the central nervous system37.
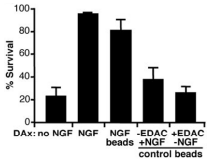
Compartmented cultures were deprived of NGF, and distal axons (DAx) were given the indicated treatments for 30 hours while cell bodies/proximal axons (CB/PAx) were exposed to an antibody against NGF. The nuclei were then stained with Hoechst DNA stain to assess neuronal survival. A minimum of 1000 neurons were categorized per treatment group as surviving (diffusely labeled DNA) or dying/dead (condensed fragmented DNA or unlabeled). The percentage of live neurons in each treatment group (±SEM, n=3 cultures) is plotted. The two bead control groups were given Native mouse NGF 2.5S protein (>95%) (#N-100) beads (50 μl/ml) prepared without EDAC cross-linking or beads (50 μl/ml) cross-linked without NGF present. Results are representative of three experiments.
Adapted from reference 44 with permission of The American Association for the Advancement of Science.
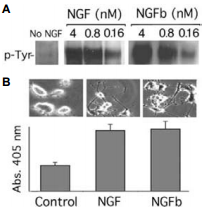
Native mouse NGF 2.5S protein (99%) (#N-240) or Native mouse NGF 2.5S protein (>95%) (#N-100) was labeled with biotin (NGFb) through the COOH moiety. A) PC12 cells were incubated with different concentrations of NGF or NGFb for 15 min, and TrkA tyrosine phosphorylation was determined. B) Morphological differentiation of PC12 cells after 3 days of treatment with 2 nM NGF or NGFb concomitant with increased acetyl cholinesterase activity as a cholinergic differentiation marker.
Adapted from reference 8 with permission of The Society for Neuroscience.
The Involvement of NGF in Pain Conditions
Tissue inflammation is typically accompanied by hyperalgesia and pain. It increases the sensitivity of high-threshold receptors, so that stimuli of lower intensity than normal can activate them. NGF is implicated in the regulation of hyperalgesia41.
Using dissociated rat dorsal root ganglion (DRG) neurons, the ability of Native mouse NGF 2.5S protein (99%) or Native mouse NGF 2.5S protein (>95%) to acutely sensitize responses of nociceptors to capsaicin or noxious heat during postnatal development was investigated. Neurons acquire sensitivity to the hyperalgesic effects of NGF between postnatal days 4 and 10 (P4 –P10). In contrast to NGF, bradykinin sensitizes responses to noxious heat in both adult and neonatal DRG neurons. These observations suggest a developmental switch in signal transduction cascades resulting in hyperalgesia during postnatal development and differences in the signaling pathways mediating bradykinin and NGF-induced sensitization66.
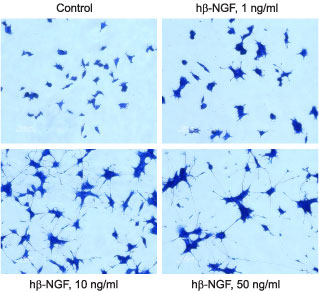
PC12 cells were grown on collagen coated 24 well plates in DMEM medium supplemented with 6% fetal calf serum and 6% horse serum for 24 h. Recombinant human beta-NGF protein (#N-245) was addded at the indicated concentrations. The culture media was changed every 2-3 days. The development of neurites over a period of 5 days was visualized by Methylene Blue staining.
References
- Alberts, P. et al. (2003) Mol. Biol. Cell 14, 4207.
- Anderson, C.N. et al. (2003) Development 130, 1123.
- Andratsch, M. et al. (2009) J. Neurosci. 29, 13473.
- Arantes, R.M. and Andrews, N.W. (2006) J. Neurosci. 26, 4630.
- Berto, G. et. al. (2007) J. Cell Sci. 120, 1859.
- Bisschops, R. et al. (2006) Am. J. Physiol. 290, G1252.
- Boesmans, W. et al. (2008) Gut 57, 314.
- Bronfman, F.C. et al. (2003) J. Neurosci. 23, 3209.
- Capsoni, S. et al. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12432.
- Carter, J.M. et al. (2003) J. Biol. Chem. 278, 44988.
- Chakrabarty, A. et al. (2008) Endocrinology 149, 3452.
- Chao, M.V. et al. (2006) Clin. Sci. (Lond). 110, 167.
- Choe, C.U. et al. (2004) J. Biol. Chem. 279, 35551.
- Cordeiro, M.L. et al. (2004) Neuropsychopharmacology 29, 39.
- Czarnecki, A. et al. (2009) J. Neurophysiol. 102, 2441.
- Darios, F. et al. (2003) Hum. Mol. Genet. 12, 517.
- Darios, F. et al. (2005) J. Neurosci. 25, 4159.
- de Chaves, E.P. et al. (2001) J. Biol. Chem. 276, 36207
- De Rosa, R. et al. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3811.
- Distler, C. et al. (2003) J. Neurophysiol. 89, 2499.
- Eshed, Y. et al. (2007) J. Cell Biol. 177, 551.
- Estacion, M. et al. (2008) J. Neurosci. 28, 11079.
- Fath, T. et al. (2002) J. Neurosci. 22, 9733.
- Fricker, F.R. et al. (2009) J. Neurosci. 29, 7667.
- Fuenzalida, K. et al. (2007) J. Biol. Chem. 282, 37006.
- Fujita, R. and Ueda, H. (2003) Cell Death Differ. 10, 1336.
- Genzen, J.R. et al. (2001) J. Neurophysiol. 86, 1773.
- Grabham, P.W. et al. (2003) J. Cell Sci. 116, 3739.
- Grabham, P.W. et al. (2007) J. Neurosci. 27, 5823.
- Greene, L.A. and Tischler, A.S. (1976) Proc. Natl. Acad. Sci U.S.A. 73, 2424.
- Grigaliunas, A. et al. (2002) J. Neurophysiol. 88, 2058.
- Hajdo-Milasinovic, A. et al. (2007) J. Cell Sci. 120, 555.
- Harrisingh, M.C. et al. (2004) EMBO J. 23, 3061.
- Kanjhan, R. et al. (2005) J. Pharmacol. Exp. Ther. 314, 1353.
- Karten, B. et al. (2003) J. Biol. Chem. 278, 4168.
- Kress, M. and Fickenscher, H. (2001) FASEB J. 15, 1037.
- Lalli, G. and Hall, A. (2005) J. Cell Biol. 171, 857.
- Lee, S.K. and Hollenbeck, P.J. (2003) J. Cell Sci. 116, 4467.
- Leffler, A. et al. (2002) J. Neurophysiol. 88, 650.
- Levi-Montalcini, R. (1987) Science 237, 1154.
- Lewin, G.R. and Mendell, L.M. (1993) Trends Neurosci. 16, 353.
- Lu, V.B. et al. (2007) J. Physiol. 584, 543.
- Lu, V.B. et al. (2009) J. Physiol. 587, 1013.
- MacInnis, B.L. and Campenot, R.B. (2002) Science 295, 1536.
- Marler, K.J. et al. (2008) J. Neurosci. 28, 12700.
- Ng, Y.P. et al. (2003) J. Biol. Chem. 278, 38731.
- Obreja, O. et al. (2005) Brain 128, 1634.
- Obreja, O. et al. (2002) FASEB J. 16, 1497.
- Okumura, F. et al. (2004) J. Biol. Chem. 279, 53533.
- Pannaccione, A. et al. (2007) Mol. Pharmacol. 72, 665.
- Perez-Gonzalez, A.P. et al. (2008) J. Physiol. 586, 4675.
- Piper, A.S. and Docherty, R.J. (2000) J. Physiol. 523, 685.
- Rathee, P.K. et al. (2002) J. Neurosci. 22, 4740.
- Saavedra, L. et al. (2007) J. Biol. Chem. 282, 35722.
- Shepherd, S.P. and Holzwarth, M.A. (2001) Am. J. Physiol. 280, C61.
- Stolovich, M. et al. (2002) Mol. Cell. Biol. 22, 8101.
- Uldry, M. et al. (2004) EMBO J. 23, 531.
- Vanden Berghe, P. et al. (2004) Am. J. Physiol. 286, G671.
- Verwer, R.W. et al. (2002) FASEB J. 16, 54.
- Visochek, L. et al. (2005) J. Neurosci. 25, 7420.
- Waetzig, V. and Herdegen, T. (2003) J. Biol. Chem. 278, 567.
- Widmer, M. et al. (2005) Endocrinology 146, 4727.
- Wiesmann, C. and de Vos, A.M. (2001) Cell. Mol. Life Sci. 58, 748.
- Williams, R.S. et al. (2002) Nature 417, 292.
- Yamamoto, N. et al. (2007) J. Biol. Chem. 282, 2646.
- Zhu, W. et al. (2004) J. Neurophysiol. 92, 3148.