CNTF, a member of the cytokine family, was originally described as a neuronal survival factor in the chick cilia ganglia. Later on it was found to have trophic effects on different types of neurons. CNTF effects are mediated by a tripartite receptor complex consisting of two signal-transducing subunits (leukemia inhibitory factor receptor (LIFR), gp130) and a CNTF-specific ligand-binding-subunit (CNTFRα) anchored to the membrane by a glycosyl-phosphatidylinositol (GPI) linkage which lacks the intracellular signaling moiety. It can be cleaved from the GPI anchor to yield an active soluble form of the receptor (sCNTFRα). CNTF binds to CNTFRα, the latter associates with gp130 which recruits LIFR and subsequently leads to the activation of a signaling cascade. This brief paper aims at summarizing the use of Alomone Labs CNTF products in the literature.
CNTF and Astrocytes
Ciliary neurotrophic factor (CNTF), a member of the interleukin-6 (IL-6) family of cytokines, is produced by astrocytes following brain injury6. CNTF directly supports the survival of a variety of neuronal populations6. In addition, CNTF activates astrocytes, promoting their capacity to support neurons and oligodendroglia2. Indeed, a study showed that Recombinant rat CNTF protein-stimulated (#C-245) astrocytes released a trophic factor(s) that doubled the number of oligodendrocyte progenitor cell (OPCs) within 48 h (Figure 1), indicating that CNTF treatment likely stimulated the release of an OPC survival promoting activity from astrocytes2. These results suggest that the production and release of CNTF during recovery from demyelination, likely enhances CNS remyelination indirectly through its actions on astrocytes2. A similar study demonstrated that CNTF specifically promoted oligodendroglial differentiation/maturation of adult hippocampus-derived Neural Progenitor Cells (NPCs) without inhibiting astrogenesis or affecting cell proliferation or cell death. Indeed, maturation of NPCs was reflected by an increase in oligodendroglia-maturation transcription factor (GTX/Nkx6.2) following CNTF treatment8. Therefore, Alomone Labs rat CNTF is commonly used in studies for the purpose of maintaining cell survival, and is added as a supplement in the growth media1,3,9.
Transgenic mice studies have shown that CNTF is required to maintain motor neurons after birth because CNTF knockout mice develop a progressive loss and atrophy of motor neurons and exhibit reduced muscle strength in adulthood7.
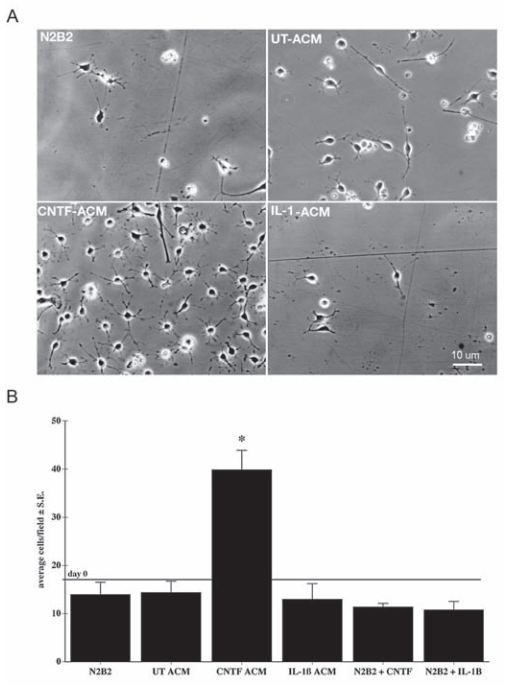
Oligodentrocyte progenitor cells (OPCs) are stimulated and proliferate when Recombinant rat CNTF protein (#C-245) is added to the growth media.
Adapted from reference 2 with kind permission of Prof. Steven W. Levison, Department of Neurology & Neurosciences. Rutgers New Jersey Medical School, NJ, USA.
CNTF in Microglia
Microglia can function as support cells for neurons to ensure neural survival or could function as phagocytes to remove degenerating cells. As IL-6, a close relative of CNTF influences the outcome of microglia, the possible effect of CNTF on microglia was investigated in two related studies4,5. It was found that rat CNTF promoted neuronal survival by recruiting microglia to increase their production of trophic factors. It did so by reducing cyclooxygenase 2 (COX-2) mRNA and protein levels and by increasing glial cell-line derived neurotrophic factor (GDNF) mRNA and protein secretion. It was also found that motor neuron survival increased when the neurons were maintained in medium conditioned by CNTF-stimulated microglia4. In a related study, treatment of microglia with Alomone Labs rat CNTF with the soluble form of CNTFRα raised central and peripheral immune responses and led to a more inflamed environment, counteracting its trophic activity on neurons and oligodendrocytes5. In that study, CNTF served as a weak pro inflammatory signal to enhance the production of Cox-2, prostaglandin E2 (PGE2) and CD40 in microglia.
Alomone Labs is pleased to offer the following CNTF-related products: Recombinant human CNTF protein (#C-240), Recombinant human Cardiotrophin-1 protein (#C-200), Recombinant human LIF protein (#L-200), Anti-CNTF Antibody (#ANT-031), and Anti-CNTFRα (extracellular) Antibody (#ACR-051).
References
- Albrecht, D. et al. (2012) Mol. Cell Neurosci. 49, 364.
- Albrecht, P.J. et al. (2007) Neurochem. Res. 32, 263.
- Jeong, G.B. et al. (2011) J. Neurosci. 31, 295.
- Krady, J.K. et al. (2008) J. Neurosci. Res. 86, 1538.
- Lin, H.W. et al. (2009) J. Neuroinflammation 6, 7.
- MacLennan, A.J. et al. (1996) J. Neurosci. 16, 621.
- Masu, Y. et al. (1993) Nature 365, 27.
- Rivera, F.J. et al. (2008) J. Neurochem. 107, 832.
- Xiong, G. et al. (2007) Eur. J. Neurosci. 25, 1987.