Genes encoding T-type CaV channels yield three pore-forming subunits: CaV3.1, CaV3.2 and CaV3.3 which give rise to currents that are pharmacologically and electrophysiologically different from the high voltage CaV1 and CaV2 channel currents. Due to their important physiological roles, and to the increasing number of channelopathies associated with T-type channels, the need of specific and potent pharmacology is rising. In this piece, we highlight the use of TTA-A2 and TTA-P2, two specific and potent T-type channel blockers exclusively available at Alomone Labs.
Introduction
T-type CaV channels regulate neuronal excitability, hormone secretion, and neurotransmitter release. They also play important roles in the circadian cycle, cardiovascular and rennin-angiotensin systems.
Knockout of CaV3.1, CaV3.2 or CaV3.3 channels in mice yields viable phenotypes, albeit each with various problems. Channelopathies associated with T-type channels include autism, epilepsy, hypertension, hyperaldosteronism, chronic pain, and neuropathic pain. Due to their important physiological roles, and to the increasing number of channelopathies associated with the channels, the need of specific and potent pharmacology is rising9.
TTA-P2
TTA-P2, a derivative of 4-aminomethyl-4-fluoropiperidine, was first discovered to potently and reversibly block T-type currents from rat acutely dissociated DRGs, with an IC50 of 100 nM and stabilize T-type channels in their inactivated states. L-type CaV channels and NaV channels are 100-1000 fold less sensitive to the compound’s blocking actions1.
TTA-P2 (#T-155) was used to gain more insight on the specific role and identity of CaV channels expressed in adrenal fasciculate cells. Indeed, TTA-P2 inhibited CaV3.2 currents expressed in the cells (Figure 1) and inhibited adrenocorticotropic hormone (ACTH) and Angiotensin II-stimulated cortisol secretion2. Under hypoxia, catecholamine secretion by the adrenal gland increases in a BDNF/TrkB-dependent manner. TrkB receptor is highly expressed in the medulla as shown in immunohistochemical staining using Alomone Labs Anti-TrkB (extracellular) Antibody (#ANT-019), and its expression increased under hypoxic conditions. In addition to catecholamine secretion, activation of TrkB also lead to an increase in [Ca2+] caused by T-type CaV currents which was inhibited by the application of TTA-P2, thus showing a linkage between BDNF/TrkB signaling and T-type currents7.
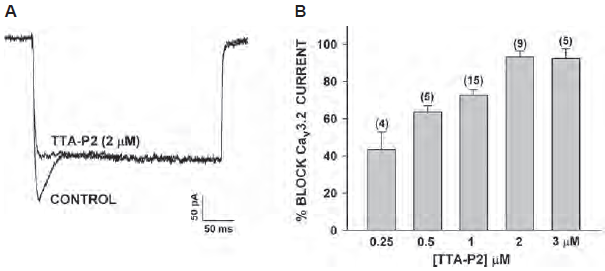
A. Bovine adrenal zona fasciculate (AZF) whole cell recordings. Ca2+ currents were recorded in 10 mM Ba2+ in response to voltage steps to -5 mV, applied from a holding potential of -80 mV, before and after superfusion of the cell with 2 μM TTA-P2 (#T-155).
B. Concentration-dependent inhibition of CaV3.2 currents by TTA-P2. Values are means ± SE for number of determinations shown in parentheses.
Adapted from reference 2 with permission of the American Physiological Society.
The transmission of pain begins at the spinal dorsal horn which then transmits the information onward. Rebound depolarization following hyperpolarization in dorsal horn neurons is an important feature in those cells and was characterized in part by the use of TTA-P2. Data show that rebound depolarization and firing by T-type CaV channels and their currents are important for integrating somatosensory information in the spinal cord6.
The thalamus plays an important role in integrating inputs from the cortex. Once the thalamus receives these inputs, it sends them back to the cortex creating a cortico-thalamo-cortical loop. These synapses and their contribution to higher brain functions were studied. Barrel cortex layer 5B neurons and those of the posteriomedial nucleus (POm) were used to study the thalamo-cortical synapse formation. Using conditional knockout of GluA4, shRNA targeting CaV3.1 channel and the T-type specific blocker TTA-P2, data show that GluA4 and CaV3.1 control important aspects of the synaptic transmission at L5-POm synapse8.
TTA-A2
TTA-A2 was discovered in an effort to identify T-type specific and potent blockers. The compound was characterized on transfected HEK 293 cells and on native T-type currents. Data show that TTA-A2 has an IC50 value of 100 nM, and like TTA-P2, it binds preferentially to CaV3 channels in their inactive state. TTA-A2 is 300-fold selective for CaV3 channels. in vivo, TTA-A2 administration suppresses active wake and promotes slow-wave sleep in wild-type but not in mice lacking CaV3.1 and CaV3.34.
Oxygen levels in the blood are detected by carotid bodies. Voltage-gated Ca2+ channels play an important role in detecting O levels. CaV3.2 channel is the major T-type CaV channel expressed in glomus cells, the cells important for sensing O2. Indeed, RT-PCR and immunohistochemistry using Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025) shows that CaV3.2 is highly expressed in the rat carotid body. Western blot analysis of rat DRG lysates using the same antibody shows that the Ca2+ channel is also expressed in DRGs. Importantly, use of the control peptide antigen completely obliterated the signal obtained with the antibody. In addition, CaV3.2 was found to be involved in mediating the carotid body’s response to hypoxia, an effect inhibited by the application of TTA-A2 (#T-140) (Figure 2)5.
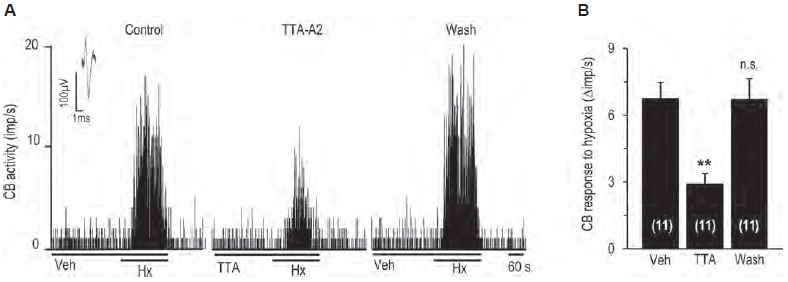
A. Representative example of sensory nerve response (impulses (imp)/s) to hypoxia in the presence of vehicle or 25 μM TTA-A2 (#T-140), and 5 min after washout.
B. Effect of TTA-A2 on sensory nerve response to hypoxia.
Adapted from reference 5 with permission of the American Physiological Society.
The contribution of T-type channels in myogenic reactivity of retinal arterial vessels was studied in arterioles of the rat retinal microcirculation. Immunohistochemical staining of rat retinal arterioles using Anti-CACNA1G (CaV3.1) Antibody (#ACC-021) and Anti-CaV3.2 (CACNA1H) Antibody showed that while CaV3.2 was not detected, strong CaV3.1 staining was observed on the plasma membrane of retinal arteriole smooth muscle cells. CaV3.2 was detected on glial cell end-feet surrounding the vessels. Application of TTA-A2 or ML 218 (#M-165) dilated isolated, myogenically active retinal arterioles, demonstrating that CaV3.1 channels are functionally expressed on arteriole smooth muscle cells or retinal arterioles and play an important role in myogenic signaling3.
References
- Choe, W. et al. (2011) Mol. Pharmacol. 80, 900.
- Enyeart, J.J. and Enyeart, J.A. (2015) Am. J. Physiol. 308, C899.
- Fernandez, J.A. et al. (2015) Invest. Ophtalmol. Vis. Sci. 56, 5125.
- Kraus, R.L. et al. (2010) J. Pharmacol. Exp. Ther. 335, 409.
- Makarenko, V.V. et al. (2015) Am. J. Physiol. 308, C146.
- Rivera-Arconada, I. and Lopez-Garcia, J.A. (2015) Pflugers Arch. 467, 1985.
- Scott, A.L. et al. (2015) J. Physiol. 593, 3281.
- Seol, M. and Kuner, T. (2015) Eur. J. Neurosci. 42, 3033.
- Zamponi, G.W. et al. (2015) Pharmacol. Rev. 67, 821.