Introduction
α-Latrotoxin is a 130 kD protein toxin from the black widow spider venom and is the only protein in the venom that affects mammals (for reviews see references 1,2).
Application of the toxin to presynaptic preparations induces, after a delay, a huge increase in spontaneous neurotransmitter release, which can be evaluated by measuring the postsynaptic response in the form of miniature end plate potentials.
It is widely used to induce and study neurotransmitter release (see Table 1), but the molecular mechanism of its action is not fully determined.
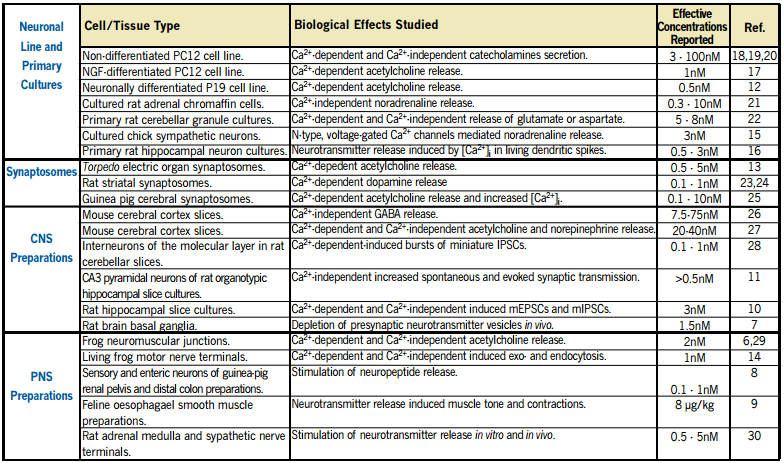
A Complex Mode of Action
Although α-Latrotoxin can form tetramers which function as cation-selective pores in membranes4, it also binds to two types of receptors on presynaptic nerve endings: Neurexin and Latrophillin (CIRL). Both receptors are essential for the toxin pore formation mechanism (membrane integration) but not for the toxin induced exocytosis2.
The binding of α-Latrotoxin to neurexin is a Ca2+-dependent reaction while its binding to CIRL is Ca2+-independent (see Figure 1A). Cation-selective pores formed by α-Latrotoxin can induce release by allowing Ca2+ to enter into the terminal, similar to voltage-dependent Ca2+ channels, forming a Ca2+-dependent release pathway (which is essential for triggering release in non-classical synapses that release monoamines or peptides). However, in classical synapses (transmitting GABA, glutamate or Ach) release is achieved also in Ca2+ free conditions, forming a Ca2+ -independent release pathway (see Figure 1B). Taking all these results into account, the following sequence of events was suggested as a model for α-Latrotoxin action on presynaptic nerve terminals. The toxin binds to its receptors, which facilitate its insertion into the plasma membrane3,5. This forms both a Ca2+ permeable channel and an intracellular N-terminal that interacts with the fusion machinery complex. The former is capable of triggering transmission in any synapse including release of peptides and monoamines, while the latter bypasses the Ca2+ requirement for fusion to directly enable release which is seen only in classical synapses (GABA, glutamate and Ach)1.
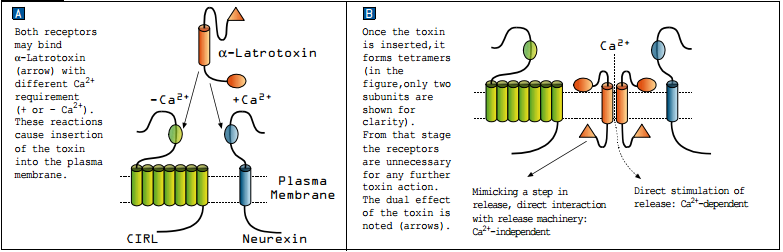
References
- Sudhof, T. C. (2001) Annu. Rev. Neurosci. 24, 933.
- Ushkaryov, Y. (2002) Toxicon 40, 1.
- Ichtchenko, K. et al. (1998) EMBO. J. 17, 6188.
- Orlova, E. V. et al. (2000) Nature Structural Biol. 7, 48.
- Volynski, K. E. et al. (2000) J. B. C. 275, 41175.
- Valtorta, F. et al. (1988) J. Cell Biol. 107, 2717.
- Herrera-Marschitz, M. et al. (1996) J. Neurochem. 66, 1726.
- Waterman, S.A. and Maggi, C.A. (1995) Neuroscience 69, 977.
- Ny, L. et al. (1997) Br. J. Pharmacol. 120, 31.
- Capogna, M. et al. (1996) J. Neurophysiol. 75, 2017.
- Capogna, M. et al. (1996) J. Neurophysiol. 76, 3149.
- Parnas, D. and Linial, M. (1995) Int. J. Dev. Neurosci. 13, 767.
- Linial, M. et al. (1995) Eur. J. Neurosci. 7, 742.
- Henkel, A.W. and Betz, W.J. (1995) Neuropharmacology 34, 1397.
- Boehm, S. and Huck, S. (1996) Eur. J. Neurosci. 8, 1924.
- Segal, M. (1995) J. Physiol. 486, 283.
- Ireland, L.M. et al. (1995) J. Pharmacol. Exp. Ther. 275, 1453.
- Watanabe, O. et al. (1983) Neuroscience 10, 1011.
- Grasso, A. et al. (1980) Nature 283, 774.
- Cattaneo, A. and Grasso, A. (1986) Biochemistry 25, 2730.
- Kobayashi, H. et al. (1986) Neurosci. Lett. 65, 114.
- Grasso, A. and Mercanti-Ciotti, M.T. (1993) Neuroscience 54, 595.
- Meldolesi, J. (1982) J. Neurochem. 38, 1559.
- Knipper, M. et al. (1986) Neuroscience 19, 55.
- Deri, Z. et al. (1993) J. Neurochem. 60, 1065.
- Tzeng, M.C. et al. (1978) P.N.A.S. USA 75, 4016.
- Tzeng, M.C. and Siekevitz, P. (1978) Brain Res. 139, 190.
- Auger, C. and Marty, A. (1997) Neuron 19, 139.
- Torri-Tarelli, F. et al. (1990) J. Cell Biol. 110, 449.
- Picotti, G.B. et al. (1982) Naun. -Schm. Arch. Pharmacol. 320, 224.
- Frontali, B. et al. (1976) J. Cell Biol. 68, 462.
- Grasso, A. (1976) B.B.A. 439, 406.