Exploring the Significance of MANF Expression in Brain Damage Detection
Throughout this blog series, we’ve delved into the significance of mesencephalic astrocyte-derived neurotrophic factor (MANF), focusing on its role in epilepsy, Parkinson’s disease, and Alzheimer’s disease. In these previous posts, we explored the expression of MANF in astrocytes in models with experimentally-induced acute damage targeting specific sites in brain. As we’ve seen, astrocytes in these models consistently expressed MANF, which leads to an intriguing question: What if the expression of MANF in astrocytes is so sensitive, that it can serve as a marker for pathological sites that develop with no experimental intervention?
FVB/N Mice: A Test Case for Immunohistochemical Pathology Markers
FVB/N mice are an inbred strain of laboratory mice commonly used in genetic research thanks to their high reproductive performance and large litter sizes. The zygotes of fertilized FVB/N eggs contain large and prominent pronuclei, which facilitate DNA microinjection, making them ideal for transgenic experiments. Notably, inbred strains like FVB/N mice may exhibit abnormal behavior due to their genetic homogeneity and gradual effects of aging. Specifically, some FVB/N mice exhibit hyperactivity and circling (running in circles), which could indicate pathological processes to neural systems that modulate locomotor behavior. Understanding these processes in FVB/N mice may contribute to the understanding of locomotor hyperactivity in some human psychiatric disorders. Astrocytes, known for their reactivity to pathological processes, express proteins that may serve as sensitive markers for the site(s) of pathology.
MANF Expression in the FVB/N Mouse Hippocampus
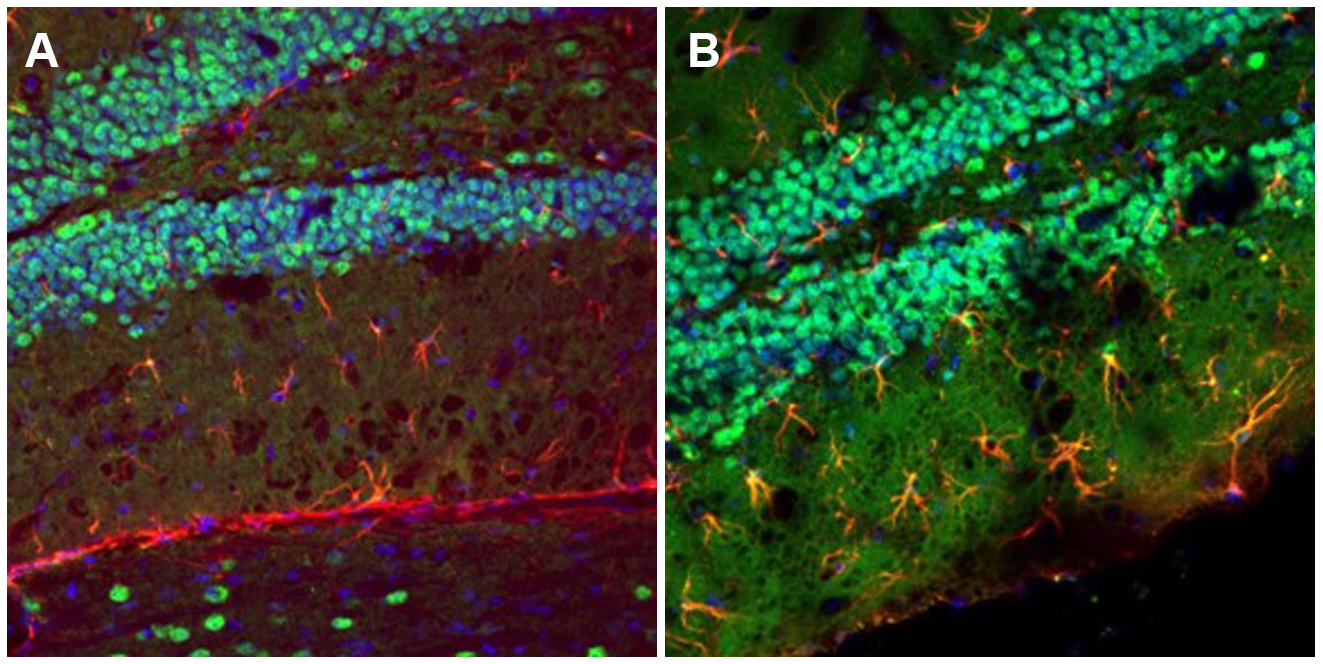
Dr. Shai Shoham, a senior member of the Alomone team, sought to transform MANF expression into a novel brain damage marker. To do so, he used Alomone’s cutting-edge antibodies for MANF and the classic astrocyte marker, glial fibrillary acidic protein (GFAP), to stain brain sections of FVB/N mice. Interestingly, he found that MANF was only expressed by astrocytes in the hippocampal dentate gyrus (DG) in hyperactive FVB/N mice, suggesting pathological processes in that area. This finding may pave the way for a great deal of further research on both the dentate gyrus’ role in abnormal behavior and MANF’s potential as a marker.
The Importance of MANF in the FVB/N Mouse Hippocampus
The hippocampus is a complex brain structure that is widely considered critical for memory formation, spatial navigation, and regulation of emotion. Much less is known about the potential involvement of the hippocampus in locomotor hyperactivity. However, the hippocampus can modulate locomotor behavior through its output to the basal ganglia. This output is mediated largely by glutamatergic transmission. The kainate epilepsy model demonstrates excess glutamatergic transmission, and as we’ve seen, MANF is overexpressed in this model.
MANF Expression in the FVB/N Mouse Dentate Gyrus
Helping you explore astrocyte reactions to brain pathology
We’ve been producing and validating a range of antibodies in-house for decades. If you enjoyed the research discussed here, you might be interested in these primary antibodies:
- Anti-MANF/ARMET Antibody (#ANT-028)
- Anti-GFAP Antibody (#AFP-001) [for GFAP expression in rats]
- Anti-Connexin-43 Antibody (#ACC-201)
- Anti-Connexin-37 Antibody (#ACC-204)
- Anti-LPAR4 (P2Y9) (extracellular) Antibody (#ALR-034)
- Anti-α1-Syntrophin (SNTA1) Antibody (#APZ-021)
- Anti-SGLT1 (extracellular) Antibody (#AGT-031)
We also have blocking peptides to be used as controls for each of these antibodies. These are the original antigens we used for immunization during antibody creation:
- MANF/ARMET Blocking Peptide (#BLP-NT028)
- GFAP Blocking Peptide (#BLP-FP001)
- Connexin-43 Blocking Peptide (#BLP-CC201)
- Connexin-37 Blocking Peptide (#BLP-CC204)
- LPAR4/P2Y9 (extracellular) Blocking Peptide (#BLP-LR034)
- α1-Syntrophin/SNTA1 Blocking Peptide (#BLP-PZ021)
- SGLT1 (extracellular) Blocking Peptide (#BLP-GT031)
