Immunocytochemistry (ICC) Protocols for Fixed or Live Cells: Indirect and Direct Methods
Detailed cell preparation and direct and indirect ICC methods.
Immunocytochemistry (ICC) allows you to locate a specific protein within or on a cell with the aid of an antibody that recognizes a particular epitope.
Indirect ICC uses a secondary antibody conjugated to a reporter. This secondary antibody binds the primary antibody. Even though this indirect method requires more steps and materials compared to the direct method, it benefits from signal amplification as multiple secondary antibodies – and their reporters –bind to the primary antibody. However, indirect ICC methods can also produce more background than direct ICC methods.
Direct ICC uses a single primary antibody to target your protein of interest. Here, the primary antibody is conjugated to a reporter such as an ATTO fluorophore. Since the direct method doesn’t require a secondary antibody, it is quicker, cheaper, and may result in less non-specific binding. However, the signal may appear weaker than the signal from indirect ICC methods, especially with proteins that have low expression levels.
- First, determine if you need to fix your cells.
- Next, chose either indirect (Option A) or direct (Option B) ICC methods.
If you have any problems, please see our extensive troubleshooting guides.
ICC Protocols for Fixed Cells
Cell Preparation
- Plate the cells in chamber slides and allow them to grow for 1–2 days in an appropriate medium. Cells need to attach strongly to the plate.
Note: some cell lines will need a special coating, e.g., polylysine on the chamber slides, to aid in cell attachment. The specific type of coating needs to be determined empirically as it varies between chamber types and cell lines. - Wash the cells 3 times with ice-cold ICC phosphate-buffered saline (ICC-PBS).
| ICC-PBS (pH 7.4) | |
|---|---|
| Reagent | Concentration |
| Na2HPO4 | 0.016 M |
| KH2PO4 | 0.003 M |
| NaCl | 0.14 M |
Fixation
- Fix the cells by adding 1–4% paraformaldehyde (PFA) in ICC-PBS and incubate for 10 minutes at room temperature.
Note: the optimal PFA concentration depends on the cell type and you need to establish this experimentally. - Wash the cells 3 times with ice-cold ICC-PBS.
Permeabilization and Blocking
- Permeabilize the cell membranes by adding Saponin Assay Buffer and incubate for 10 minutes at room temperature.
| Saponin Assay Buffer | |
|---|---|
| Reagent | % of final volume |
| ICC-PBS | 97.9 |
| Bovine serum albumin (BSA) | 2 |
| Saponin | 0.1 |
- Block the non-specific binding sites with 5% normal serum (NS)* in Saponin Assay Buffer for 15 minutes at room temperature.
*Use a serum based on the species that your secondary antibody was raised in. For example, if your secondary antibody was raised in donkeys, use normal donkey serum (NDS). Likewise, if your secondary antibody was raised in goats, use normal goat serum (NGS).
Proceed to EITHER indirect (A) or direct (B) labeling methods.
OPTION A: Indirect Labeling of Fixed Cells
- Add the primary antibody at the appropriate dilution in Saponin Assay Buffer and incubate for 1–2 hours at 4°C.
- Wash the cells 3 times with Saponin Assay Buffer.
- Add the fluorophore-conjugated secondary antibody at the appropriate dilution in Saponin Assay Buffer and incubate for 1 hour at 4°C, protected from light.
- Add DAPI (5 mg/ml stock solution in deionized water or dimethylformamide (DMF); dilute to 500 nM in ICC-PBS for final use) and incubate for 5 minutes at room temperature.
- Wash 3 times with Saponin Assay Buffer and drain well.
- Add ICC-PBS to cover the cells and proceed with microscopy.
OPTION B: Direct Labeling of Fixed Cells Using ATTO-Conjugated Primary Antibodies
- Add the ATTO-conjugated primary antibody at the appropriate dilution in Saponin Assay Buffer.
- Incubate for 1 hour at 4°C, protected from light.
- Add DAPI (5 mg/ml stock solution in deionized water or dimethylformamide (DMF); dilute to 500 nM in ICC-PBS for final use) and incubate for 5 minutes at room temperature.
- Wash 3 times with Saponin Assay Buffer and drain well.
- Add ICC-PBS to cover the cells and proceed with microscopy.
ICC Protocols for Live Cells
Cell Preparation
- Plate the cells in chamber slides and allow them to grow for 1–2 days in an appropriate medium. Cells need to attach strongly to the plate.
Note: some cell lines will require a special coating, e.g., polylysine on the chamber slides, to aid in cell attachment. The specific type of coating needs to be determined empirically as it varies between chamber types and cell lines. - Wash the cells 3 times with ice-cold Assay Buffer.
| ICC-PBS (pH 7.4) | |
|---|---|
| Reagent | Concentration |
| Na2HPO4 | 0.016 M |
| KH2PO4 | 0.003 M |
| NaCl | 0.14 M |
| Assay Buffer | |
|---|---|
| Reagent | % of final volume |
| ICC-PBS | 97.95 |
| Bovine serum albumin | 2 |
| NaN3 | 0.05 |
Proceed to EITHER indirect (A) or direct (B) labeling methods.
OPTION A: Indirect Labeling of Live Cells
- Add the primary antibody at the appropriate dilution in ice-cold Assay Buffer.
- Incubate for 1–2 hours at 4°C.
- Wash the cells 3 times with ice-cold Assay Buffer.
- Add the fluorophore-conjugated secondary antibody at the appropriate dilution in ice-cold Assay Buffer and incubate for 1 hour at 4°C protected from light.
- Add DAPI (5 mg/ml stock solution in deionized water or dimethylformamide (DMF); dilute to 500 nM in ICC-PBS for final use) and incubate for 5 minutes at room temperature.
- Wash 3 times with ice-cold Assay Buffer and drain well.
- Add ICC-PBS to cover the cells and proceed with microscopy.
OPTION B: Direct Labeling of Live Cells Using ATTO-Conjugated Primary Antibodies
- Add the ATTO-conjugated primary antibody at the appropriate dilution in ice-cold Assay Buffer.
- Incubate for 1 hour at 4°C.
- Add DAPI (5 mg/ml stock solution in deionized water or dimethylformamide (DMF); dilute to 500 nM in ICC-PBS for final use) and incubate for 5 minutes at room temperature.
- Wash 3 times with ice-cold Assay Buffer and drain well.
- Add ICC-PBS to cover the cells and proceed with microscopy.
Example Data
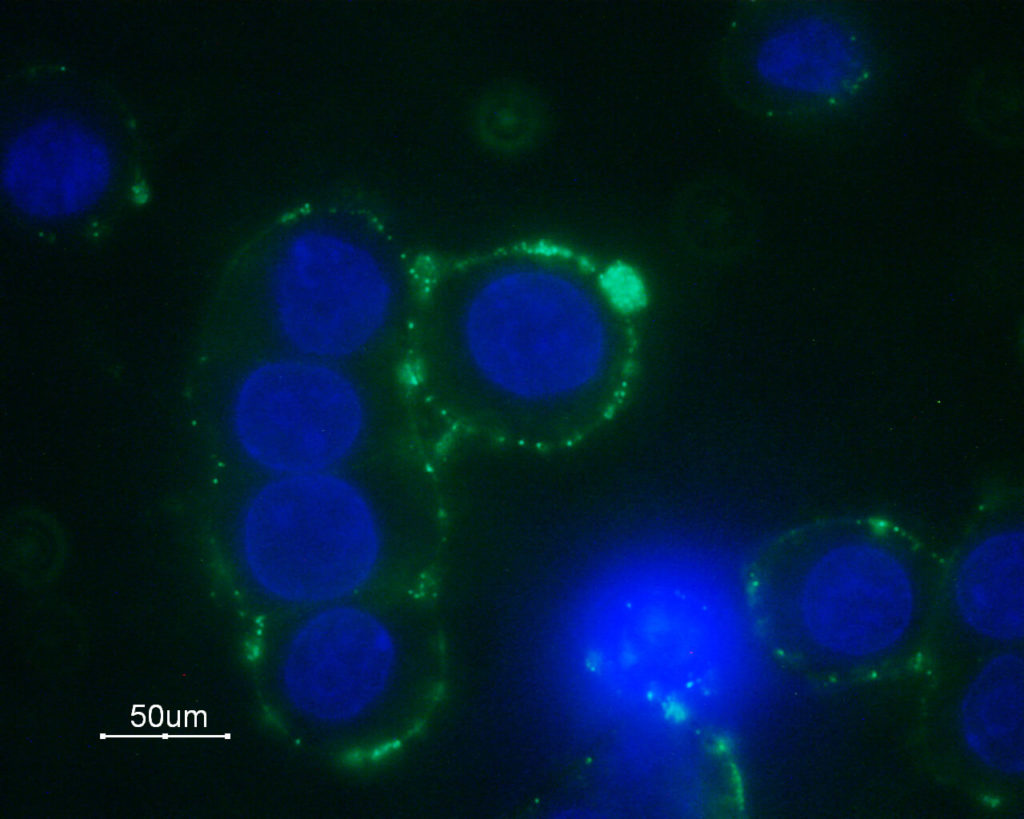
Expression of the α1B-adrenoceptor in GH3 cells. Cell surface detection of α1B-adrenoceptor in living GH3 cells. The cells were stained with Anti-α1B-Adrenergic Receptor (extracellular) Antibody (AAR-018) (1:100) followed by staining with goat anti-rabbit Alexa Fluor 488 secondary antibody (green). The cell nuclei were stained with the DNA dye Hoechst 33342 (blue).
Expression of the noradrenaline transporter (NET) in live intact rat pheochromocytoma (PC12) cells. Cell surface detection of NET in live intact rat PC12 cells. The cells were stained with Anti-Noradrenaline Transporter (extracellular) Antibody (AMT-002) (1:100) followed by staining with goat anti-rabbit Alexa Fluor 594 secondary antibody (red). The cell nuclei were visualized using the cell-permeable DNA dye Hoechst 33342 (blue).
Expression of KCNQ1 in Human COLO-205 cells. Cell surface detection of KCNQ1 in live intact human COLO-205 colon adenocarcinoma cells. A. Extracellular staining of cells with Anti-KCNQ1 (extracellular) Antibody (APC-168), (1:50), followed by goat anti-rabbit-AlexaFluor-488 secondary antibody (green). B. Live view of the cells. C. Merge of A and B.
Expression of Kir4.1 in human U-87 MG cells. Cell surface detection of Kir4.1 in live intact human glioblastoma U-87 MG cells. A. Extracellular staining of cells using Anti-Kir4.1 (KCNJ10) (extracellular) Antibody (APC-165), (1:100) followed by goat anti-rabbit-AlexaFluor-594 secondary antibody (red). B. Live image of the cells. C. Merge of A and B.