TDP-43 accumulation disrupts local protein synthesis in axons and NMJs, which reduces axonal and synaptic levels of mitochondrial proteins and sensitizes NMJs to rapid degeneration. On the other hand, could TDP-43 clearance represent an attractive future therapeutic target to reverse the effects of ALS?
Amyotrophic lateral sclerosis (ALS) is a terrifying and debilitating disease. Typically affecting adults, ALS is characterized by degeneration of motor neurons (MNs) and disruption of neuromuscular junctions (NMJs). Sadly, people with ALS rarely survive more than 3–4 years. One of the hallmark pathologies of ALS is mislocalization and accumulation of the TAR-DNA-binding-protein-43 (TDP-43) in the cytoplasm of MNs, but how exactly this contributes to the disease is largely unknown.
Recent work from Israel’s Tel Aviv University, led by Topaz Altman and Ariel Ionescu et al., and published in Nature Communications, has revealed a great deal more about the role of TDP-43 in ALS.1
A better model to understand TDP-43 at the NMJ
What we do know is that as TDP-43 accumulates, you see phase-separated cytoplasmic condensates and the development of ribonucleoprotein (RNP) condensates, which in turn deregulate mRNA localization and translation. The combination of RNP condensate formation and the disruption to mRNA transport upset local protein synthesis, which seems to be essential to maintaining axonal and synaptic health. While existing models support the idea of altered protein synthesis being relevant to ALS, much of the previous work has been conducted in non-neuronal cells or within the neuronal cell body, rather than the more appropriate NMJs.
Altman and Ionescu wanted to address this specific issue. So, they used a neuromuscular co-culture in custom-made microfluidic chambers (MFCs) to more accurately model pathological features of ALS, like axon degeneration, NMJ dysfunction, and MN death. This custom setup also meant they could look at local protein synthesis at the subcellular level.
The researchers said they were “confident that these large and remote synaptic structures [NMJs] must depend on local protein synthesis in order to maintain their proper function and support local mitochondria.” Combined with what’s already known about the relatively lower capacity of axons to handle stress compared to cell bodies, Altman and Ionescu said they were “intrigued to understand if and how the previously reported TDP-43-mediated stress to cells would take action in axons and NMJs.”
Studying the NMJ with co-cultures
One of the biggest challenges in studying NMJs comes from their spatial complexity and the difficulty in detecting the specific axonal and presynaptic signals. To overcome these issues, the researchers used advanced cell-culture and co-culture approaches, such as puromycin-resistant muscles for visualizing only pre-synaptic protein synthesis, alongside mitochondrial reporter mice. Altman and Ionescu said that “The almost surgical ability that co-cultures can be used and manipulated in microfluidics chambers, complement and give stable grounds to our in-vivo results and strengthen our findings overall.”
TDP-43 behavior in the axons
The first steps in this research were to confirm and explore the behavior of TDP-43 in their custom system, ideally with ALS biopsies. But getting muscle biopsies from early symptomatic ALS patients is challenging ad most ALS studies that use human samples use post-mortem ones, which typically only show late-stage ALS pathologies. What the team needed were samples of the early stage. Thankfully, the researchers didn’t need to rely on cadavers for their muscle biopsies: “We were able to form a fruitful collaboration with the Neuromuscular Diseases Clinic at the Sheba Tel-Hashomer hospital, led by Dr. Amir Dori, who collected muscle biopsies from patients during their initial diagnosis, which gave us a window to study the early pathological events happening in ALS patients,” said Altman and Ionescu.
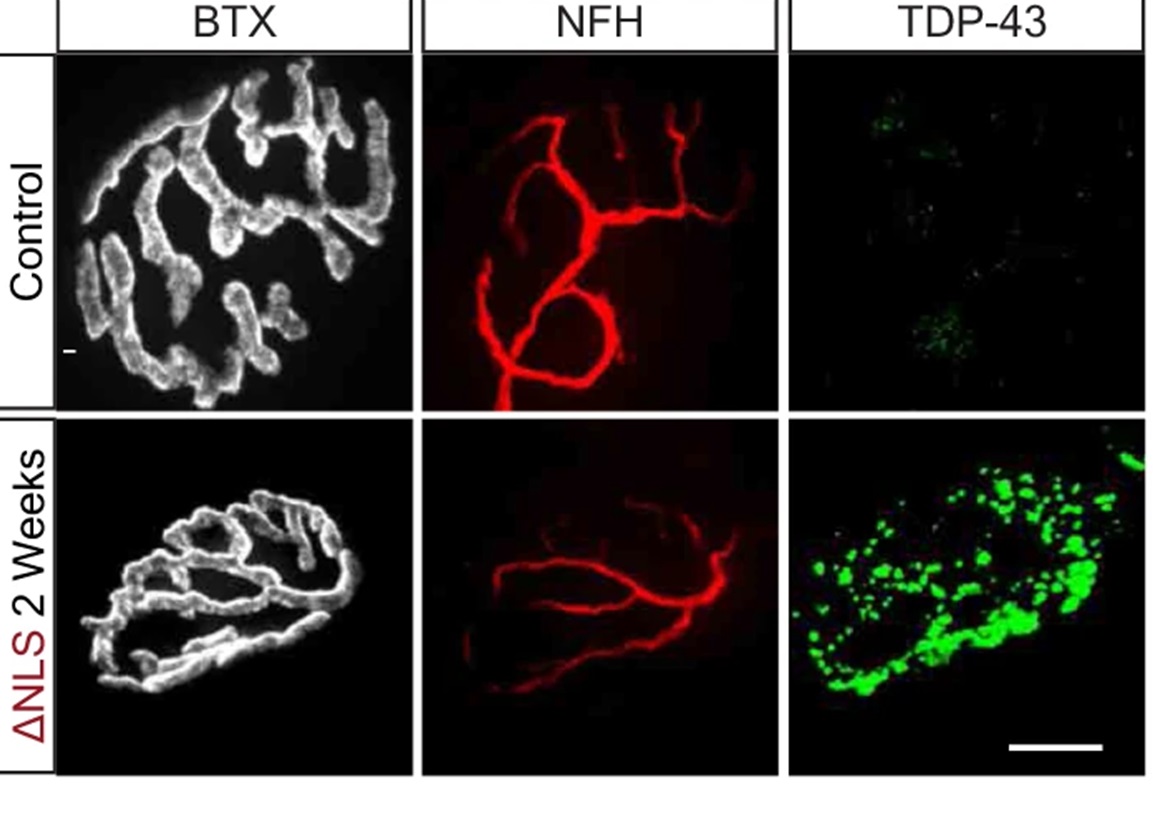
So, with access to these tissues, they stained TDP-43 muscle biopsies from ALS and non-ALS patients to reveal that TDP-43 is elevated in intra-muscular nerves and that pathological phosphorylated TDP-43 (pTDP-43) accumulates in MN axons of ALS patients (a result confirmed in mouse models) (Figure 1). Their experiments here also showed that TDP-43 leads to the formation of RNP condensates in MN axons that contain RNA and GTPase-activating protein-binding-protein1 (G3BP1).
Upsetting local protein synthesis
It turns out that it’s these RNA- and G3BP1-containing RNP condensates that interfere with local protein synthesis in the axons and the NMJs. Mechanistically, TDP-43 mislocalization binds and sequesters nuclear-encoded mitochondrial mRNAs, which disrupts protein synthesis and depletes their level of MN axonal proteins (Figure 2).
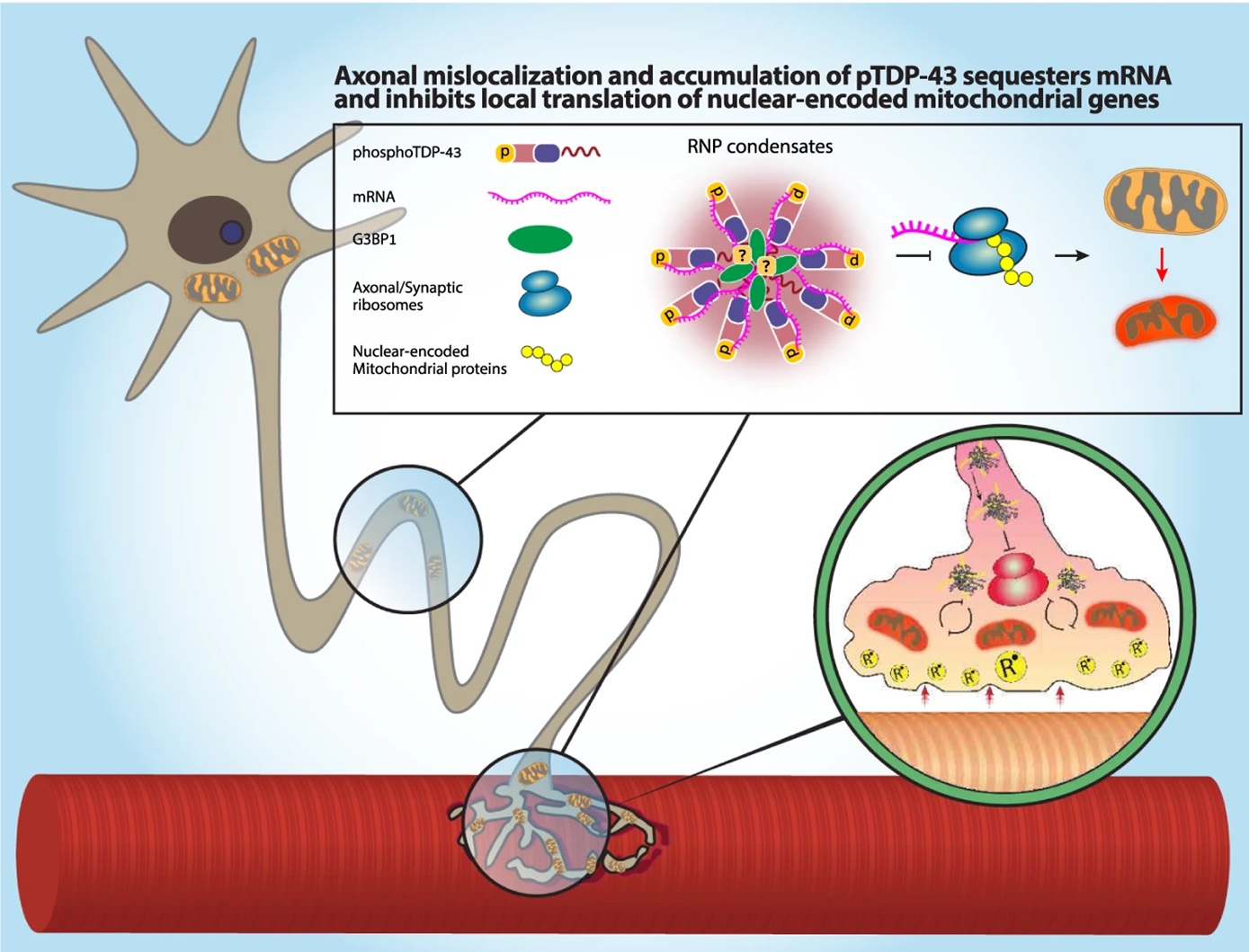
To arrive at this conclusion, they first confirmed that pre-synaptic mitochondria were essential to maintaining NMJ activity before then examining whether local protein synthesis was similarly critical for NMJs. By adding puromycin to the NMJ compartment in co-cultures with puromycin-resistant muscles they inhibited protein synthesis only in presynaptic axons. Looking at calcium transients in the post-synaptic muscles showed a decrease in active muscles, which sufficiently confirms that local protein synthesis is indeed needed for functional NMJs.
But what happens if that local protein synthesis is disrupted? With mitochondria and local protein synthesis neatly shown to be essential for NMJ activity, the researchers went on to see what happens after inhibition of protein synthesis in MN axons. Looking at these MNs over time in the presence of protein synthesis inhibitors, it became clear that NMJs undergo extensive degeneration.
Removal of TDP-43 improves axon and NMJ function
The direct presence of TDP-43 raised the question as to whether the mislocalization of TDP-43 and its associated decrease in protein synthesis and subsequent degeneration be alleviated if you removed it? To test this, they used inducible transgenic mice that express the human TDP-43 but which lack the nuclear-localization signal (ΔNLS) through the doxycycline (dox) TET-off system. This TDPΔNLS model was able to reproduce ALS pathologies like NMJ disruption.
By stopping TDP-43ΔNLS expression the researchers saw a reduction in axonal TDP-43 and pTDP-43 in the spinal cord and clearance of synaptic condensates from NMJs. They managed to show that the altered mitochondrial protein turnover, caused by TDP-43 mislocalization and disruption of axonal and synaptic protein synthesis, was reversible upon the clearance of TDP-43 RNP condensates.
Pivotal findings and an exciting future
This remarkable work not only ties together the TDP-43 mislocalization seen in ALS with the disruption of mitochondria-related protein synthesis that induces neurodegeneration, but it also highlights the role of local protein synthesis. Since NMJs depend on both mitochondria activity on local protein synthesis, any interference with synaptic protein synthesis can cause a local energy deficiency, eventually resulting in NMJ degeneration. The work here suggests the mechanism behind NMJ degeneration seen in ALS is a TDP-43-mediated reduction in local protein synthesis and a subsequent sensitization of the NMJs to degeneration.
But there’s more. In addition to adding to our understanding of ALS, the researchers have shown that TDP-43 clearance may be a useful therapeutic option and how peptides that interfere with G3BP1-TDP-43 RNP condensates may also offer beneficial results. And any new therapy options for ALS patients is an exciting future indeed.
The lab now plans to identify the basic mechanisms that regulate local protein synthesis in NMJs as well as the role of TDP-43 interactions with RNA in axons and NMJs plays in other diseases. “We are also very interested in understanding if and how muscles regulate presynaptic local protein synthesis in NMJs and are currently investigating whether they do so by transmitting miRNA and mRNA loaded exosomes trans-synaptically.”
Alomone reagents used in the research
Postsynaptic marker
For fluorescent stainings, the team used the labeled toxin, α-Bungarotoxin-ATTO Fluor-633 (#B-100-FR) to mark the postsynaptic compartments.
Neurotrophic factors
The team also made extensive use of cell culture that included multiple growth factors from Alomone Labs. In the generation of ALS patient-derived induced pluripotent stem cell (iPSC) MNs, they made use of Recombinant human Neurotrophin-3 (NT-3) protein (#N-260). In their MN culture and MN-myocyte co-culture, Recombinant human BDNF protein (#B-250) was used for spinal cord explants. The MNs were maintained in a complete neurobasal medium that contained both Recombinant human GDNF protein (#G-240) and Recombinant human CNTF protein (#C-240) amongst other essential reagents. All of our in-house-made and tested growth factors are in excess of 98% purity (confirmed by HPLC) and have ultra-low endotoxin levels (<0.1 EU per 1 µg of the protein as determined by the LAL method).
Reference
Photo by Pawel Czerwinski.
