Overview
- Wilson, S.P. and Kirshner, N. (1977) J. Neurochem. 28, 687.
- Garcia-Guzman, M. et al. (1995) Eur. J. Neurosci. 7, 647.
- McCann, C.M. et al. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5149.
Centrifuge all products BEFORE adding solvent (10,000 x g for 5 minutes). The preparation of fresh solutions in working buffers before use is recommended. Soluble in pure water to high-micromolar concentrations (50µM-1mM). For long-term storage in solution, it is recommended to prepare a stock solution by dissolving the product in double distilled water (ddH2O) at a concentration between x100-1000 of the final working concentration. Divide the solution into single-use aliquots and store at -20°C. Before use, thaw the relevant vial(s) intended for use and dilute in the desired working buffer. Avoid multiple freeze-thaw cycles to maintain toxin activity.
 Live cell imaging of α-Bungarotoxin-ATTO Fluor-633 in differentiated PC-12 cells.Neurite outgrowth was induced in PC12 cells through seven days exposure to 100 ng/ml Native mouse NGF 2.5S protein (>95%) (#N-100). (A) CellMask™ Actin 1X solution was applied for 30 minutes, resulting in a green fluorescence to visualize cellular membrane. (B) Following this, the same cells underwent incubation with 0.5 µM of α-Bungarotoxin-ATTO Fluor-633 for 30 minutes at 37ºC, followed by PBSX1 wash, leading to red fluorescence indicative of the distribution of nicotinic ACh receptors channels. (C) Live imaging of the differentiated PC-12 cells allowed observation of α-Bungarotoxin distribution among the cells.
Live cell imaging of α-Bungarotoxin-ATTO Fluor-633 in differentiated PC-12 cells.Neurite outgrowth was induced in PC12 cells through seven days exposure to 100 ng/ml Native mouse NGF 2.5S protein (>95%) (#N-100). (A) CellMask™ Actin 1X solution was applied for 30 minutes, resulting in a green fluorescence to visualize cellular membrane. (B) Following this, the same cells underwent incubation with 0.5 µM of α-Bungarotoxin-ATTO Fluor-633 for 30 minutes at 37ºC, followed by PBSX1 wash, leading to red fluorescence indicative of the distribution of nicotinic ACh receptors channels. (C) Live imaging of the differentiated PC-12 cells allowed observation of α-Bungarotoxin distribution among the cells. Expression of α-Bungarotoxin-ATTO Fluor-633 in mouse cortexImmunohistochemical staining of perfusion-fixed frozen mouse brain sections with Anti-Nicotinic Acetylcholine Receptor α7 (CHRNA7) (extracellular) Antibody (#ANC-007), followed by goat-anti-rabbit-AlexaFluor-568, followed by α-Bungarotoxin-ATTO Fluor-633, 0.8 µM. Staining in mouse cortex shows co-staining of apical dendrites (arrow) of pyramidal neurons with α-Bungarotoxin-ATTO Fluor-633 and CHRNA7. Cell nuclei are stained with DAPI (blue).
Expression of α-Bungarotoxin-ATTO Fluor-633 in mouse cortexImmunohistochemical staining of perfusion-fixed frozen mouse brain sections with Anti-Nicotinic Acetylcholine Receptor α7 (CHRNA7) (extracellular) Antibody (#ANC-007), followed by goat-anti-rabbit-AlexaFluor-568, followed by α-Bungarotoxin-ATTO Fluor-633, 0.8 µM. Staining in mouse cortex shows co-staining of apical dendrites (arrow) of pyramidal neurons with α-Bungarotoxin-ATTO Fluor-633 and CHRNA7. Cell nuclei are stained with DAPI (blue). Alomone Labs α-Bungarotoxin-ATTO Fluor-633 in whole mount staining of mice neuromuscular junction (NMJ)Whole mount staining of mouse neuromuscular junction (NMJ) was stained with the NMJ marker α-Bungarotoxin-ATTO Fluor-633 (#B-100-FR) (purple) at 1 µg/ml concentration. The image was taken using Nikon Epifluorescence microscopy at 60X magnification and is kindly provided by Dr. Eran Perlsson, Dept. of Physiology and Pharmacology, Tel-Aviv University, Tel-Aviv, Israel.
Alomone Labs α-Bungarotoxin-ATTO Fluor-633 in whole mount staining of mice neuromuscular junction (NMJ)Whole mount staining of mouse neuromuscular junction (NMJ) was stained with the NMJ marker α-Bungarotoxin-ATTO Fluor-633 (#B-100-FR) (purple) at 1 µg/ml concentration. The image was taken using Nikon Epifluorescence microscopy at 60X magnification and is kindly provided by Dr. Eran Perlsson, Dept. of Physiology and Pharmacology, Tel-Aviv University, Tel-Aviv, Israel. Direct flow cytometry of α-Bungarotoxin in live intact THP-1 monocyte cells___ THP-1 cells.
Direct flow cytometry of α-Bungarotoxin in live intact THP-1 monocyte cells___ THP-1 cells.
___ THP-1 cells + 1 µM α-Bungarotoxin (#B-100).
___ THP-1 cells + 1 µM α-Bungarotoxin-ATTO Fluor-633 (#B-100-FR). Direct flow cytometry of α-Bungarotoxin in live intact rat PC-12 cells___ PC-12 cells.
Direct flow cytometry of α-Bungarotoxin in live intact rat PC-12 cells___ PC-12 cells.
___ PC-12 cells + 1 µM α-Bungarotoxin (#B-100).
___ PC-12 cells + 1 µM α-Bungarotoxin-ATTO Fluor-633 (#B-100-FR).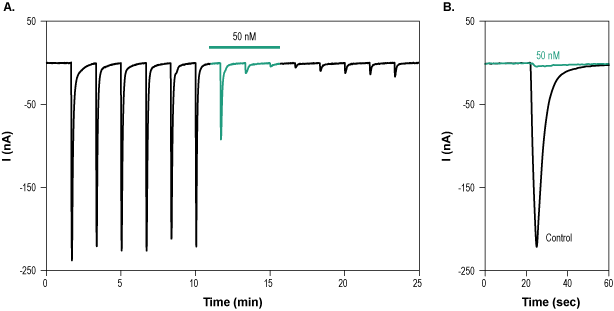 Alomone Labs α-Bungarotoxin-ATTO Fluor-633 inhibits muscle nACh receptors heterologously expressed in Xenopus oocytes.A. Time course of muscle nicotinic ACh channel current recording. Membrane potential was held at -80 mV and stimulated every 100 sec with a solution containing 100 µM ACh + 3 μM PNU-120596. 50 nM α-Bungarotoxin-ATTO Fluor-633 (#B-100-FR) was applied (green) for 5 min during the ACh application and inhibited channel current. B. Superimposed examples of muscle nicotinic ACh current in the absence (black) and presence (green) of 50 nM α-Bungarotoxin-ATTO Fluor-633 (taken from the experiment in A).
Alomone Labs α-Bungarotoxin-ATTO Fluor-633 inhibits muscle nACh receptors heterologously expressed in Xenopus oocytes.A. Time course of muscle nicotinic ACh channel current recording. Membrane potential was held at -80 mV and stimulated every 100 sec with a solution containing 100 µM ACh + 3 μM PNU-120596. 50 nM α-Bungarotoxin-ATTO Fluor-633 (#B-100-FR) was applied (green) for 5 min during the ACh application and inhibited channel current. B. Superimposed examples of muscle nicotinic ACh current in the absence (black) and presence (green) of 50 nM α-Bungarotoxin-ATTO Fluor-633 (taken from the experiment in A).
- Ohta, M. et al. (1987) FEBS Lett. 222, 79.
- Wilson, P.T. et al. (1988) Mol. Pharmacol. 34, 643.
- Wilson, S.P. and Kirshner, N. (1977) J. Neurochem. 28, 687.
- Garcia-Guzman, M. et al. (1995) Eur. J. Neurosci. 7, 647.
- McCann, C.M. et al. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5149.
α-Bungarotoxin isoform A31 is a 74 amino acid peptidyl toxin isolated from the venom of the banded krait snake, Bungarus multicinctus1.
α-Bungarotoxin blocks postsynaptic neuromuscular transmission via competitive inhibition of nicotinic ACh receptors (nAChRs) with an IC50 of 3.5 x 10-10 M, thereby preventing the depolarizing action on postsynaptic membranes and blocking neuromuscular transmission2.
The toxin is selective for α7 receptors (IC50 value of 1.6 nM) and α3/β4 receptors (IC50 value of >3 µM)3,4.
α-Bungarotoxin also binds to and blocks a subset of GABAA receptors (GABAARs) that contain the GABAAR β3 subunit. In particular, α-Bungarotoxin blocks GABAARs that contain interfaces between adjacent β3 subunits5.
α-Bungarotoxin-ATTO Fluor-633 (#B-100-FR) is a highly pure, natural, and biologically active conjugated peptide toxin.
Benefits of α-Bungarotoxin-ATTO Fluor-633:
✓ Localization and distribution
✓ Clustering and internalization kinetics
✓ Live cell imaging
✓ Single cell detection
✓ Binding kinetics
We gladly take on collaboration projects. Please Contact Us.
Applications
Citations
- Altman, T., et al. (2021) Nat Commun., 12, 6914.

