Overview
α-Conotoxin AuIB is a 15 amino acid peptidyl toxin isolated from Conus aulicus1. This toxin inhibits mammalian neuronal α3/β4 nicotinic acetylcholine receptor (nAChR) channels expressed in Xenopus oocytes with an IC50 of 0.75 µM. Furthermore, α-Conotoxin AuIB blocks the α3/β4 receptor with >100-fold higher potency than other receptor subunit combinations1.
In accordance, α-Conotoxin AuIB (1-5 µM) blocks 20-35% of the nicotine-stimulated norepinephrine release from rat hippocampal synaptosomes1 and the nicotinic ACh receptors on dissociated neurons of the rat parasympathetic ganglia with IC50 of 1.2 nM2.
α-Conotoxin AuIB also mediates inhibition of N-type calcium channels at nanomolar concentrations (IC50 = 1.5 nM in rat DRG neurons) via agonism of γ-aminobutyric acid (GABA) G protein-coupled (GABAB) receptors3. GABAB receptors are promising targets for the treatment of various neurological and psychiatric disorders, including pain, depression, and drug addiction. GABAB receptors are widely expressed and distributed in pain-processing pathways at all levels of the neuraxis and play an extensive role in editing and modulating nociceptive inputs. α-Conotoxin AuIB produced reversal of allodynia in the partial nerve ligation (PNL) model3,4.
Specifications
Technical Specifications
Biological Activity
Solubility and Storage
Applications
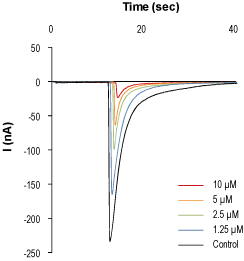 Alomone Labs α-Conotoxin AuIB inhibits α3/β4 nAChR channels heterologously expressed in Xenopus oocytes.Superimposed traces of α3/β4 nAChR channel current in the absence (black) and presence of different concentrations of α-Conotoxin AuIB (#STC-310). Current amplitudes were plotted as a function of time. Membrane potential was held at -60 mV and oocytes were stimulated by exposure to 20 μM Acetylcholine and 1 μM Atropine, for 1 second every 50 seconds. The indicated different α-Conotoxin AuIB concentrations (inset) were perfused during the period marked in colors.
Alomone Labs α-Conotoxin AuIB inhibits α3/β4 nAChR channels heterologously expressed in Xenopus oocytes.Superimposed traces of α3/β4 nAChR channel current in the absence (black) and presence of different concentrations of α-Conotoxin AuIB (#STC-310). Current amplitudes were plotted as a function of time. Membrane potential was held at -60 mV and oocytes were stimulated by exposure to 20 μM Acetylcholine and 1 μM Atropine, for 1 second every 50 seconds. The indicated different α-Conotoxin AuIB concentrations (inset) were perfused during the period marked in colors.

