Overview
- Jin, A.H. et al. (2014) Biochemistry 53, 1.
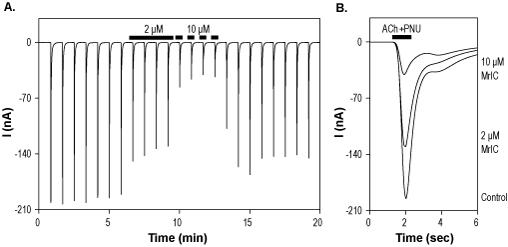 Alomone Labs α-Conotoxin MrIC inhibits α7 nAChR heterologously expressed in Xenopus oocytes.A. Time course of α-Conotoxin MrIC (#STC-320) action on α7 nAChR currents, elicited every 50 sec by a transient applications of 100 µM ACh + 0.3 μM PNU-120596, while membrane potential was held at -80 mV. Application of 2 µM and 10 µM α-Conotoxin MrIC (as indicated by bars) significantly inhibits the currents. B. Superimposed traces of α7 nAChR currents upon application of control, 2 µM and 10 µM α-Conotoxin MrIC (taken from the experiment in A).
Alomone Labs α-Conotoxin MrIC inhibits α7 nAChR heterologously expressed in Xenopus oocytes.A. Time course of α-Conotoxin MrIC (#STC-320) action on α7 nAChR currents, elicited every 50 sec by a transient applications of 100 µM ACh + 0.3 μM PNU-120596, while membrane potential was held at -80 mV. Application of 2 µM and 10 µM α-Conotoxin MrIC (as indicated by bars) significantly inhibits the currents. B. Superimposed traces of α7 nAChR currents upon application of control, 2 µM and 10 µM α-Conotoxin MrIC (taken from the experiment in A).
- Jin, A.H. et al. (2014) Biochemistry 53, 1.
- Mueller, A. et al. (2015) Biochem. Pharmacol. 94, 155.
- Lustig, L.R. (2006) Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288, 424.
- McIntosh, J.M. et al. (2009) Biochem. Pharmacol. 78, 693.
α-Conotoxin MrIC is peptide toxin originally isolated from Conus marmoreus. It is a competitive and selective antagonist of α7 nicotinic acetylcholine receptor (nAChRα7). Peptide toxins belonging to α-conotoxin family are relatively short peptides, between 12−19 amino acid residues and demonstrate high affinity towards nAChRs. They are stabilized by two disulfide bonds1,2.
In Ca2+ responses assay to α-Conotoxin MrIC in SH-SY5Y cells endogenously expressing α7 nAChR, α-Conotoxin MrIC elicits concentration-dependent increases in intracellular Ca2+ in the presence of the α7 nAChR-specific positive allosteric modulator PNU120596 with an EC50 value of 1.9 µM2. Interestingly, α-Conotoxin MrIC antagonizes nAChRα7 heterologously expressed in Xenopus oocytes1.
nAChRs receptors are responsible for mediating the effects of the neurotransmitter acetylcholine (ACh). They play critical physiologic roles in the central and peripheral nervous system where they regulate neurotransmitter release, cell excitability, neuronal integration, and are involved in functions such as sleep and arousal patterns, fatigue, hunger, anxiety, and pain processing3,4.
α-Conotoxin MrIC (#STC-320) is a highly pure, synthetic, and biologically active peptide toxin.

