Overview
- Hopkins, C. et al. (1995) J. Biol. Chem. 270, 22361.
- Teichert, R.W. et al. (2006) Biochemistry 45, 1304.
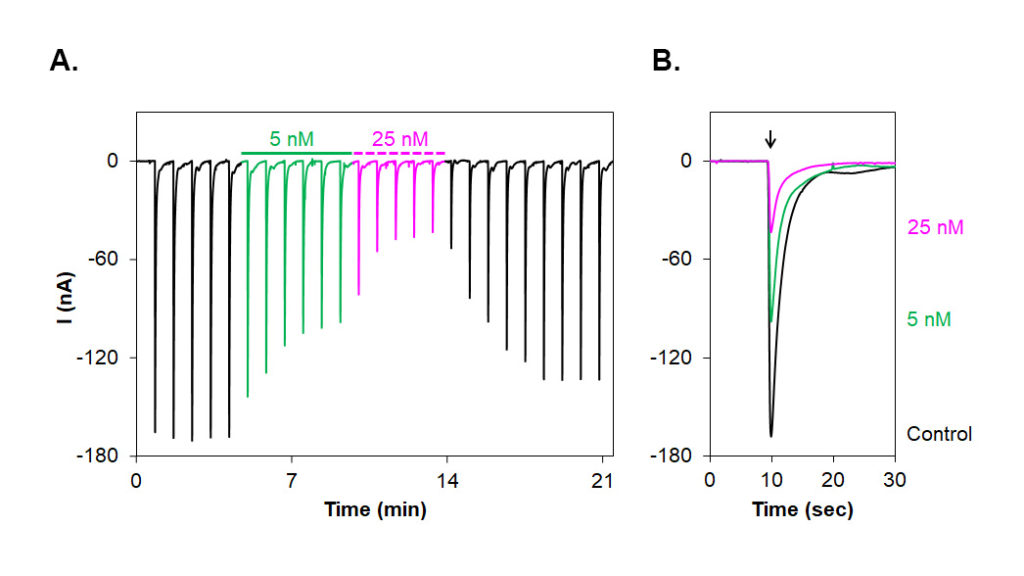 Alomone Labs αA-Conotoxin PIVA inhibits muscle fetal α1/β1/γ/δ nAChR heterologously expressed in Xenopus oocytes.A. Time course of αA-Conotoxin PIVA (#STC-600) action on muscle α1/β1/γ/δ nAChR. Current amplitudes were plotted as a function of time. Membrane potential was held at –80 mV and oocytes were stimulated by exposure to 10 µM acetylcholine every 50 seconds. 5 nM (green) and 25 nM (magenta) αA-Conotoxin PIVA significantly inhibits the currents.
Alomone Labs αA-Conotoxin PIVA inhibits muscle fetal α1/β1/γ/δ nAChR heterologously expressed in Xenopus oocytes.A. Time course of αA-Conotoxin PIVA (#STC-600) action on muscle α1/β1/γ/δ nAChR. Current amplitudes were plotted as a function of time. Membrane potential was held at –80 mV and oocytes were stimulated by exposure to 10 µM acetylcholine every 50 seconds. 5 nM (green) and 25 nM (magenta) αA-Conotoxin PIVA significantly inhibits the currents.
B. Superimposed traces of α1/β1/γ/δ nAChR currents evoked by ACh (arrow) after application of control, 5 nM (green) and 25 nM (magenta) αA-Conotoxin PIVA (taken from the recording in A).
αA-Conotoxin PIVA is the first member of a new family of nAChR-targeted Conus peptides, named short αA-conotoxins1. It is a peptide toxin originally isolated from Conus Purpurascens (Purple cone) venom1.
This peptide reversibly blocks postsynaptic muscle fetal α1/β1/γ/δ nicotinic ACh receptor1,2 . It also blocks postsynaptic muscle adult α1/β1/ε/δ nicotinic ACh receptor, with lower affinity (IC50 of 22 nM to the adult compared to 2.3 nM to the fetal nAChR)2.
αA-conotoxin PIVA has a strikingly different amino acid sequence and a Cys framework from all other nAChR-targeted peptides previously characterized from Conus venoms. Despite the striking structural divergence, the peptide blocks the ACh binding site of the nAChR at the neuromuscular junction, similar to the well known typical of a-conotoxins1.
The characterization of the short αA-conotoxins revealed diverse kinetics of a block of the fetal muscle nAChR, particularly in dissociation rates. The structure-function relationships of native αA-conotoxins revealed a single amino acid locus (alternatively either His or Pro) that is a critical determinant of the dissociation kinetics2.

