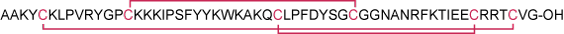Overview
Cat #:
D-380
Lyophilized Powder yes
Origin Natural peptide isolated from Dendroaspis angusticeps (Eastern green mamba).
MW: 6568 Da
Purity: >98% (HPLC)
Effective concentration 10-100 nM.
Sequence AAKYCKLPVRYGPCKKKIPSFYYKWKAKQCLPFDYSGCGGNANRFKTIEECRRTCVG.
Modifications Disulfide bonds between Cys5-Cys55, Cys14-Cys38 and Cys30-Cys51.
Molecular formula C296H458N82O76S6.
Activity δ-Dendrotoxin inhibits 4-AP sensitive, inactivating voltage-gated K+ channels (KV1.1, KV1.2 and KV1.6). In addition δ-Dendrotoxin inhibits ROMK1 channel currents.
Shipping and storage Shipped at room temperature. Product as supplied can be stored intact at room temperature for several weeks. For longer periods, it should be stored at -20°C.
Solubility Any aqueous buffer. Centrifuge all product preparations before use (10000 x g 5 min).
Storage of solutions Up to four weeks at 4°C or three months at -20°C.
Our bioassay
 Alomone Labs δ-Dendrotoxin blocks KV1.1 channels expressed in Xenopus oocytes.A. Representative time course of δ-Dendrotoxin (#D-380) inhibition of KV1.1 current. Membrane potential was held at -100 mV, current was elicited by a 100 ms voltage ramp to +20 mV every 10 sec, and significantly inhibited by 0.5 nM δ-Dendrotoxin (green). B. Superimposed traces of KV1.1 current following application of control (black) and of 0.5 nM δ-Dendrotoxin (green), taken from the recording in A.
Alomone Labs δ-Dendrotoxin blocks KV1.1 channels expressed in Xenopus oocytes.A. Representative time course of δ-Dendrotoxin (#D-380) inhibition of KV1.1 current. Membrane potential was held at -100 mV, current was elicited by a 100 ms voltage ramp to +20 mV every 10 sec, and significantly inhibited by 0.5 nM δ-Dendrotoxin (green). B. Superimposed traces of KV1.1 current following application of control (black) and of 0.5 nM δ-Dendrotoxin (green), taken from the recording in A.
Scientific background
δ-Dendrotoxin is isolated from Dendroaspis angusticeps1 snake venom by modification of the procedures of Harvey2 and Benishin1 and purified to homogeneity.
δ-Dendrotoxin blocks KV1.1 channels (IC50 = 0.1 nM,3 for review see 4). The toxin was shown to also block KV1.6 (IC50= 23 nM).4 In addition δ-Dendrotoxin blocks ROMK1 inward rectifier K+ channels with Kd of 150 nM.3
Target KV1.1, KV1.6 and Kir1.1 K+ channels
Peptide Content: 100%
Lyophilized Powder
For research purposes only, not for human use
Last Update: 08/01/2025
Specifications
Citations
Citations