Overview
Cat #:
STC-760
Lyophilized Powder yes
Origin Synthetic peptide
MW: 2640 Da
Purity: >98% (HPLC)
Effective concentration 1-10 nM.
Sequence CKGKGASCRRTSYDCCTGSCRSGRC-NH2
Modifications Disulfide bonds between Cys1-Cys16, Cys8-Cys20 and Cys15-Cys25. Cys25 - C-terminal amidation.
Molecular formula C98H164N40O35S6.
Activity ω-Conotoxin CVIE is a selective voltage-gated N-type Ca2+ channel inhibitor1-3.
References-Activity
- Berecki, G. et al. (2010) Mol. Pharmacol. 77, 139.
- Adams, D.J. et al. (2012) Br. J. Pharmacol. 166, 486.
- Sadeghi, M. et al. (2013) Mol. Pain 9, 51.
Accession number
Shipping and storage Shipped at room temperature. Product as supplied can be stored intact at room temperature for several weeks. For longer periods, it should be stored at -20°C.
Solubility Any other aqueous buffer. Centrifuge all product preparations before use (10000 x g 5 min).
Storage of solutions Up to two weeks at 4°C or three months at -20°C.
Our bioassay
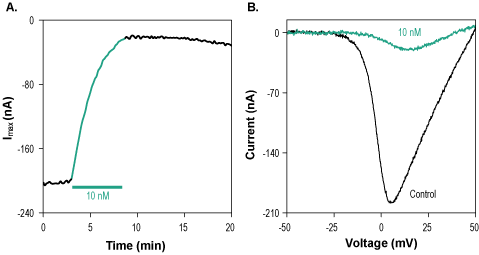 Alomone Labs ω-Conotoxin CVIE blocks CaV2.2 currents heterologously expressed in Xenopus oocytes.A. Time course of ω-Conotoxin CVIE (#STC-760) blocking action on CaV2.2 currents elicited in 2 mM Ba2+. Maximum current amplitudes were plotted as a function of time. Membrane potential was held at -100 mV and cells were stimulated by a 100 ms voltage ramp to +50 mV. 10 nM ω-Conotoxin CVIE were perfused as indicated by the bar (green) during 5 min. B. Superimposed examples of CaV2.2 channel current in the absence (control) and presence (green) of 10 nM ω-Conotoxin CVIE (taken from the experiment in A).
Alomone Labs ω-Conotoxin CVIE blocks CaV2.2 currents heterologously expressed in Xenopus oocytes.A. Time course of ω-Conotoxin CVIE (#STC-760) blocking action on CaV2.2 currents elicited in 2 mM Ba2+. Maximum current amplitudes were plotted as a function of time. Membrane potential was held at -100 mV and cells were stimulated by a 100 ms voltage ramp to +50 mV. 10 nM ω-Conotoxin CVIE were perfused as indicated by the bar (green) during 5 min. B. Superimposed examples of CaV2.2 channel current in the absence (control) and presence (green) of 10 nM ω-Conotoxin CVIE (taken from the experiment in A).
References - Scientific background
- Berecki, G. et al. (2010) Mol. Pharmacol. 77, 139.
- Adams, D.J. et al. (2012) Br. J. Pharmacol. 166, 486.
- Sadeghi, M. et al. (2013) Mol. Pain 9, 51.
Scientific background ω-Conotoxin CVIE is a peptide toxin originally isolated from the sea snail-cat cone (Conus Catus). It is a selective blocker of N-type CaV channels and has an IC50 of 2.6 nM1. It is hypothesized that CVIE mostly interacts with the extracellular part of the channels but also modulates calcium channel binding kinetics and affinity through interaction with the channel’s intracellular part and auxiliary subunits. CVIE has a stronger affinity for inactivated calcium channels. Calcium channels recover from CVIE in a voltage dependent manner; recovery is faster at strong membrane hyperpolarization. Since voltage gated calcium channels play an important role in regulating neuronal excitability and nociceptors signal transduction, CVIE has been found to be effective in the alleviation of neuropathic and inflammatory pain2. Unlike other substances that alleviate pain CVIE does not produce sedation and motor-deficit side effect in vitro but further investigation is required for in vivo data3.
Target N-Type Ca2+ channels
Peptide Content: 100%
Lyophilized Powder
ω-Conotoxin CVIE (#STC-760) is a highly pure, synthetic, and biologically active peptide toxin.
For research purposes only, not for human use
Last Update: 08/01/2025
Applications
Citations
Citations

