Overview
- Ramilo, C.A. et al. (1992) Biochemistry 31, 9919.
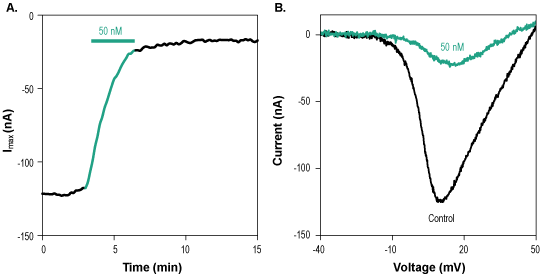 Alomone Labs ω-Conotoxin SVIB inhibits CaV2.2 currents heterologously expressed in Xenopus oocytes.A. Time course of ω-Conotoxin SVIB (#STC-160) inhibition of CaV2.2 maximum current amplitude. Membrane potential was held at -100 mV and currents were elicited by a 100 ms voltage ramp to +50 mV in 2 mM Ba2+. ω-Conotoxin SVIB was applied at 50 nM for 3 min (green). B. Superimposed traces of CaV2.2 current upon application of control (black) and of 50 nM ω-Conotoxin SVIB (green), taken from the recording shown in A.
Alomone Labs ω-Conotoxin SVIB inhibits CaV2.2 currents heterologously expressed in Xenopus oocytes.A. Time course of ω-Conotoxin SVIB (#STC-160) inhibition of CaV2.2 maximum current amplitude. Membrane potential was held at -100 mV and currents were elicited by a 100 ms voltage ramp to +50 mV in 2 mM Ba2+. ω-Conotoxin SVIB was applied at 50 nM for 3 min (green). B. Superimposed traces of CaV2.2 current upon application of control (black) and of 50 nM ω-Conotoxin SVIB (green), taken from the recording shown in A.
- Ramilo, C.A. et al. (1992) Biochemistry 31, 9919.
- Lee, S. et al. (2010) Mol. Pain 6, 97.
- Adams, D.J. and Berecki, G. (2013) Biochim. Biophys. Acta. 1828, 1619.
ω-Conotoxin SVIB, an analogue of ω-conotoxin GVIA, is a peptide toxin originally isolated from Conus striatus venom. It is a potent blocker of N-type voltage-gated Ca2+ channels. It has an analgesic effect and is being used as a potential drug for the treatment of chronic and neuropathic pain models1,2.
ω-Conotoxins are disulfide-rich peptides comprises 24–31 amino acids, isolated from the venom of marine predatory cone snails. These conotoxins have a high content of basic amino acid residue and a common cysteine scaffold that stabilizes the four-loop framework2,3.
Voltage-gated N-Type Ca2+ channels are predominantly expressed in nerve terminals and are involved in the regulation of neuronal excitability and nociceptive signals. These channels play an important role in the transduction of acute and chronic pain perception. They are able to transduce electrical activity into other cellular functions and regulate calcium homeostasis3.
ω-Conotoxin SVIB (#STC-160) is a highly pure, synthetic, and biologically active peptide toxin.

