Overview
- Rosker, C. et al. (2007) Am. J. Physiol. 293, C783.
 Alomone Labs 4,9-Anhydrotetrodotoxin selectively blocks NaV1.6 channels expressed in Xenopus oocytes.A. Time course of 4,9-Anhydrotetrodotoxin (#T-560) inhibition of NaV1.6 maximum current amplitude. Membrane potential was held at -100 mV and currents were elicited by a 100 ms voltage step to 0 mV every 10 sec. 4,9-Anhydrotetrodotoxin applied at 10 nM and 50 nM, as indicated (bars), reversibly inhibits NaV1.6 currents in a concentration dependent manner. B. Superimposed traces of NaV1.6 current upon application of control and of 10 nM and 50 nM 4,9-Anhydrotetrodotoxin (as indicated), taken from the recording shown in A.
Alomone Labs 4,9-Anhydrotetrodotoxin selectively blocks NaV1.6 channels expressed in Xenopus oocytes.A. Time course of 4,9-Anhydrotetrodotoxin (#T-560) inhibition of NaV1.6 maximum current amplitude. Membrane potential was held at -100 mV and currents were elicited by a 100 ms voltage step to 0 mV every 10 sec. 4,9-Anhydrotetrodotoxin applied at 10 nM and 50 nM, as indicated (bars), reversibly inhibits NaV1.6 currents in a concentration dependent manner. B. Superimposed traces of NaV1.6 current upon application of control and of 10 nM and 50 nM 4,9-Anhydrotetrodotoxin (as indicated), taken from the recording shown in A.
4,9-Anhydrotetrodotoxin, also called 4,9-anhydro-TTX, is a derivative of tetrodotoxin, a potent neurotoxin found in a number of marine creatures including the pufferfish. 4,9-Anhydro-TTX acts as a potent and selective NaV1.6 channel blocker with an IC50 value of 7.8 nM1.
4,9-Anhydrotetrodotoxin was found to block the activity of NaV1.6 channels with an efficacy of 40–160 times higher than other tetrodotoxin sensitive NaV1.x channel isoforms. The compound successfully prevents the influx of Na+ ions by blocking the pore of the NaV channel from the extracellular side of the plasma membrane2.
4,9-Anhydrotetrodotoxin has become a useful pharmacological tool for the investigation and identification of the biophysical properties of NaV1.6 channels2.
Voltage-gated sodium (NaV) channels are responsible for initiating action potentials in electrically excitable cells. NaV channels play an important role in human pain signaling pathways and are an important therapeutic target for the treatment of chronic pain. Mutations in NaV channels are also associated with a range of complex pathologic conditions such as epilepsy, myotonic conditions and cardiac arrhythmias3,4.
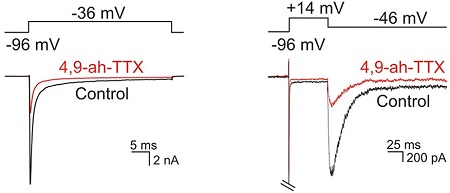
Alomone Labs 4,9-Anhydrotetrodotoxin blocks INaT, INaR and INaP in mouse SGNs through NaV1.6.Whole-cell voltage clamp recordings on mouse spiral ganglion neurons (SGNs). A depolarizing voltage step to -36 mV activated transient inward Na+ currents (INaT, black trace), which was blocked >70% by bath applied 100 nM 4,9-Anhydrotetrodotoxin (#T-560), (red, left panel). A repolarization from +14 to -46 mV activated resurgent Na+ currents (INaR) and persistent Na+ currents (INaP) (black trace), which were both blocked ~70% by bath applied 100 nM 4,9-Anhydrotetrodotoxin (red, right panel).Adapted from Browne, L. et al. (2017) eNeuro 4, e0303. with permission of the Society for Neuroscience.

