Overview
- Peptide (C)NGSRAPDHDVTQERDE, corresponding to amino acid residues 15-30 of mouse ADRB2 (Accession P18762). Extracellular, N-terminus.
- Human THP-1 monocytic leukemia and Jurkat T-cell leukemia cells (5 µg antibody/0.5x106 cells).
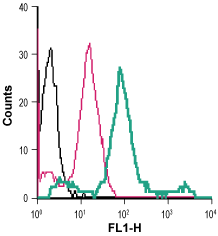 Cell surface detection of β2-Adrenoceptor in live intact human THP-1 monocytic leukemia cells:___ Cells.
Cell surface detection of β2-Adrenoceptor in live intact human THP-1 monocytic leukemia cells:___ Cells.
___ Cells + Rabbit IgG isotype control-FITC.
___ Cells + Anti-β2-Adrenergic Receptor (extracellular)-FITC Antibody (#AAR-016-F), (5 µg/0.5x106 cells).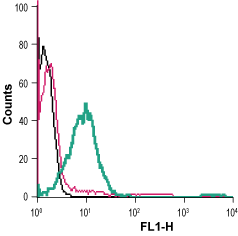 Cell surface detection of β2-Adrenoceptor in live intact human Jurkat T-cell leukemia cells:___ Cells.
Cell surface detection of β2-Adrenoceptor in live intact human Jurkat T-cell leukemia cells:___ Cells.
___ Cells + Rabbit IgG isotype control-FITC.
___ Cells + Anti-β2-Adrenergic Receptor (extracellular)-FITC Antibody (#AAR-016-F), (5 µg/0.5x106 cells).- The control antigen is not suitable for this application.
G-protein coupled receptors (GPCRs) comprise the largest protein superfamily in mammalian genomes. Signal transduction by GPCRs is fundamental for most physiological processes, spanning from vision, smell and taste to neurological, cardiovascular, endocrine, and reproductive functions, thus, making the GPCR superfamily a major target for therapeutic intervention.
The β2-adrenergic receptor (ADRB2) is comprised of seven transmembrane α-helices, connected by three extracellular loops and three intracellular loops. The extracellular part, responsible for ligand binding, also includes the N-terminus. The intracellular C-terminal interacts with G-proteins, arrestins and other downstream effectors1.
Unlike some GPCRs, ADRB2 activates more than one G-protein and signals through at least one known G-protein-independent pathway, arrestin. In addition, there is a rich diversity of available ligands for ADRB2. These ligands are often characterized as inverse agonists that suppress basal activity, full agonists that maximally activate the receptor, partial agonists that produce submaximal activity even at saturating concentrations, and neutral antagonists that occupy the orthosteric binding site but do not affect basal activity2.
ADRB2 located on the surface of cardiomyocytes, mediates distinct effects on cardiac function and the development of heart failure by regulating production of the secondary messenger cyclic adenosine monophosphate (cAMP). In cardiomyocytes from healthy adult rats and mice, spatially confined ADRB2 induces cAMP signals that are localized exclusively to the deep transverse tubules. In contrary, in cardiomyocytes derived from a rat model of chronic heart failure, ADRB2 is redistributed from the deep transverse tubules to the cell crest, which leads to diffuse receptor-mediated cAMP signaling. Thus, the redistribution of ADRB2 in heart failure might contribute to the failing myocardial phenotype3.
