Overview
|
| Bioz Stars Product Rating | |
| The world's only objective ratings for scientific research products | |
| Mentions | |
| Recency | |
| View product page > | |
Alomone Labs is pleased to offer a highly specific antibody directed against an epitope of mouse syntrophin alpha 1. Anti-α1-Syntrophin (SNTA1) Antibody (#APZ-021) can be used in western blot and immunohistochemistry applications. It has been designed to recognize syntrophin alpha 1 from mouse, rat and human samples.
Application key:
Species reactivity key:
Applications
 Western blot analysis of mouse heart lysate (lanes 1 and 3) and rat skeletal muscle membranes (lanes 2 and 4):1,2. Anti-α1-Syntrophin (SNTA1) Antibody (#APZ-021), (1:400).
Western blot analysis of mouse heart lysate (lanes 1 and 3) and rat skeletal muscle membranes (lanes 2 and 4):1,2. Anti-α1-Syntrophin (SNTA1) Antibody (#APZ-021), (1:400).
3,4. Anti-α1-Syntrophin (SNTA1) Antibody, preincubated with α1-Syntrophin/SNTA1 Blocking Peptide (#BLP-PZ021). Western blot analysis of mouse brain lysate:1. Anti-α1-Syntrophin (SNTA1) Antibody (#APZ-021), (1:200).
Western blot analysis of mouse brain lysate:1. Anti-α1-Syntrophin (SNTA1) Antibody (#APZ-021), (1:200).
2. Anti-α1-Syntrophin (SNTA1) Antibody, preincubated with α1-Syntrophin/SNTA1 Blocking Peptide (#BLP-PZ021).
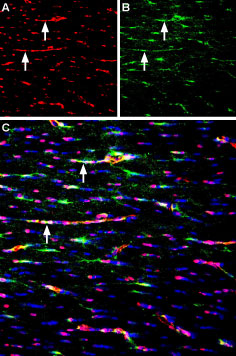
 Expression of Syntrophin alpha 1 in mouse neocortexImmunohistochemical staining of perfusion-fixed frozen mouse brain sections using Anti-α1-Syntrophin (SNTA1) Antibody (#APZ-021), (1:400), followed by anti-rabbit-AlexaFluor-488 antibody (green). Syntrophin alpha 1 staining (green) appears in blood vessels (horizontal arrows) and in the pia mater (vertical arrows). Nuclei are stained with DAPI (blue).
Expression of Syntrophin alpha 1 in mouse neocortexImmunohistochemical staining of perfusion-fixed frozen mouse brain sections using Anti-α1-Syntrophin (SNTA1) Antibody (#APZ-021), (1:400), followed by anti-rabbit-AlexaFluor-488 antibody (green). Syntrophin alpha 1 staining (green) appears in blood vessels (horizontal arrows) and in the pia mater (vertical arrows). Nuclei are stained with DAPI (blue).
Citations (4)
- Mouse spinal cord sections.
Chaboub, L.S. et al. (2016) J. Neurosci. 36, 11904.
- Mouse spinal cord sections.
Chaboub, L.S. et al. (2016) J. Neurosci. 36, 11904.
Specifications
- Peptide (C)RQPSSPGPQPRNLSE, corresponding to amino acid residues 191-205 of mouse α1-syntrophin (Accession Q61234). Intracellular, PH1 domain.

Scientific Background
α1-Syntrophin is a component of the dystrophin scaffold-protein complex that acts in recruiting proteins through their multiple protein–protein interaction motifs to the cytoplasmic side of plasma membranes. Proteins interacting with α1-syntrophin include voltage-gated sodium channels, neuronal nitric oxide synthase, calmodulin, ankyrin repeat-rich membrane spanning protein, G-proteins and more.
The syntrophin family comprises five members: α1-syntrophin, β1-syntrophin, β2-syntrophin, γ1-syntrophin, and γ2-syntrophin1.
α1-Syntrophin is a peripheral cytoplasmic membrane protein in skeletal and cardiac muscle cells1.
The α1-syntrophin structure contains four conserved domains, two pleckstrin homology domains (PH1 and PH2), a PSD95-disks large-ZO1 (PDZ) domain and a syntrophin unique (SU) COOH-terminal domain2. The pleckstrin domains are responsible for the recruitment of proteins to the sarcolemma. The PDZ domain is inserted within the PH1 domain and binds to NOS-1 in skeletal muscle. This domain also binds to the C-terminus of the Kir2.1 channel and also to NaV1.5 and so allowing them to interact. The SU domain binds syntrophin to dystrophin2,3. α1-Syntrophin is also responsible for the site-specific anchoring of AQP4 protein. α1-Syntrophin deletion leads to the disruption of the polarized subcellular expression of AQP4 in the perivascular membranes4.
Several studies have shown that syntrophin-deficiency in Duchenne muscular dystrophy can ultimately lead to muscle degeneration. Syntrophin-deficiency was also shown to reduce levels of nNOS in mice5,6.
- Bhat, H.F. et al. (2014) Br. J. Cancer. 110, 706.
- Wilde, A.A. et al. (2011) Circ. Res. 108, 884.
- Willis, B.C. et al. (2105) Am. J. Physiol. Heart. Circ. Physiol. 308, 1463.
- Anderova, M. et al. (2014) PLoS One 9, e113444.
- Williams, J.C. et al. (006) J. Biol. Chem. 281, 23341.
- Koo, T. et al. (2011) Hum. Gene. Ther. 22, 1379.
