Overview
- Peptide (C)EDYGDYWRGDYEVNGVD, corresponding to amino acid residues 197 - 213 of human ACE2 (Accession Q9BYF1). Extracellular, N-term.

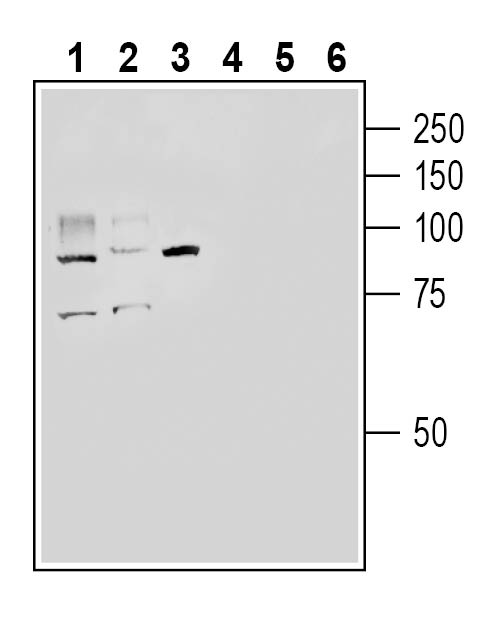 Western blot analysis of rat small intestine lysate (lanes 1 and 4), mouse lung lysate (lanes 2 and 5 ) and mouse kidney lysate (lanes 3 and 6):1-3. Anti-ACE2 (extracellular) Antibody (#AAC-012), (1:200).
Western blot analysis of rat small intestine lysate (lanes 1 and 4), mouse lung lysate (lanes 2 and 5 ) and mouse kidney lysate (lanes 3 and 6):1-3. Anti-ACE2 (extracellular) Antibody (#AAC-012), (1:200).
4-6. Anti-ACE2 (extracellular) Antibody, preincubated with ACE2 (extracellular) Blocking Peptide (BLP-AC012).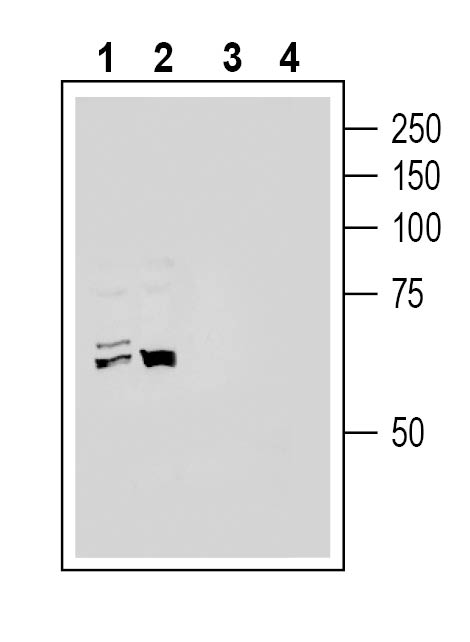 Western blot analysis of human HepG2 hepatoma cell line lysate (lanes 1 and 3) and human THP-1 monocytic leukemia cell line lysate (lanes 2 and 4):1-2. Anti-ACE2 (extracellular) Antibody (#AAC-012), (1:200).
Western blot analysis of human HepG2 hepatoma cell line lysate (lanes 1 and 3) and human THP-1 monocytic leukemia cell line lysate (lanes 2 and 4):1-2. Anti-ACE2 (extracellular) Antibody (#AAC-012), (1:200).
3-4. Anti-ACE2 (extracellular) Antibody, preincubated with ACE2 (extracellular) Blocking Peptide (BLP-AC012).
 Expression of ACE2 in mouse thalamus.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Anti-ACE2 (extracellular) Antibody (#AAC-012), (1:200), followed by goat anti-rabbit-AlexaFluor-488. A. ACE2 immunoreactivity (green) appears in neurons (arrows). B. Pre-incubation of the antibody with ACE2 (extracellular) Blocking Peptide (BLP-AC012), suppressed staining. Cell nuclei are stained with DAPI (blue).
Expression of ACE2 in mouse thalamus.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Anti-ACE2 (extracellular) Antibody (#AAC-012), (1:200), followed by goat anti-rabbit-AlexaFluor-488. A. ACE2 immunoreactivity (green) appears in neurons (arrows). B. Pre-incubation of the antibody with ACE2 (extracellular) Blocking Peptide (BLP-AC012), suppressed staining. Cell nuclei are stained with DAPI (blue).
- Wong, M. (2016). Handbook of Hormones, 263, e29D-4.
- Samavati, L. et al. (2020) Front. Cell. Infect. Microbiol., 5, 317.
- Lubbe, L. et al. (2020) Clin. Sci., 134, 2851.
The renin–angiotensin system is a central mechanism for blood pressure regulation through a diverse system of hormones and receptors. Angiotensin-converting enzyme (ACE) is an ectoenzyme that plays a role in the generation of Ang II by catalyzing the extracellular conversion of the decapeptide Ang I. Moreover, ACE degrades active bradykinin (BK), which play an important role in the control of blood pressure. ACE2 is an ACE homolog that was discovered in 2000 when two independent research groups cloned homologous ACE that could convert Ang I to Ang (1–9). Sequence analysis suggests that ACE and ACE2 exhibit 42% amino acid homology and ACE2 has evolved through gene duplication1.
ACE2 consist of N-terminal peptidase domain with seven potential N-linked glycosylation sites that bears the characteristic HEXXH motif of M2 metalloproteases. It is mainly expressed in the small intestine, testis, kidneys, heart, thyroid and adipose tissue and in the lungs. ACE 2 has a protective role in the cardiovascular system and in alveolar epithelial cells. In the lungs ACE2 has numerous physiological functions, most of which are protective against lung injury2.
ACE 2 was identified as the receptor for highly pathogenic SARS (severe acute respiratory syndrome) coronavirus including the SARS-CoV-2 that cause the coronavirus disease 2019 (COVID-19) global pandemic. SARS virus binding downregulates the cellular expression of ACE2, and the binding induces clathrin-dependent internalization of virus/receptor (SARS/ACE2) complex. Not only has ACE2 facilitated the invasion of SARS virus for rapid replication, but also ACE2 is depleted from the cell membrane and therefore the damaging effects of Ang II are enhanced, resulting in acute deterioration of lung tissues3.
Application key:
Species reactivity key:
Anti-ACE2 (extracellular) Antibody (#AAC-012) is a highly specific antibody directed against an extracellular epitope of the human protein. The antibody can be used in western blot, immunohistochemistry and flow cytometry applications. It has been designed to recognize ACE2 from rat, mouse and human samples.

