Overview
- Peptide (C)HDFSKYRQRIKQHAD, corresponding to amino acid residues 367 - 381 of mouse ADAM23 (Accession Q9R1V7). Extracellular, N-terminus.

ADAM23 (extracellular) Blocking Peptide (#BLP-NR155)
 Western blot analysis of rat brain membranes (lanes 1 and 3) and mouse brain membranes (lanes 2 and 4):1-2. Anti-ADAM23 (extracellular) Antibody (#ANR-155), (1:200).
Western blot analysis of rat brain membranes (lanes 1 and 3) and mouse brain membranes (lanes 2 and 4):1-2. Anti-ADAM23 (extracellular) Antibody (#ANR-155), (1:200).
3-4. Anti-ADAM23 (extracellular) Antibody, preincubated with ADAM23 (extracellular) Blocking Peptide (BLP-NR155). Western blot analysis of human THP-1 monocytic leukemia cell line lysate:1. Anti-ADAM23 (extracellular) Antibody (#ANR-155), (1:200).
Western blot analysis of human THP-1 monocytic leukemia cell line lysate:1. Anti-ADAM23 (extracellular) Antibody (#ANR-155), (1:200).
2. Anti-ADAM23 (extracellular) Antibody, preincubated with ADAM23 (extracellular) Blocking Peptide (BLP-NR155).
 Expression of ADAM23 in mouse cerebellum.Immunohistochemical staining of perfusion-fixed frozen mouse brain sections with Anti-ADAM23 (extracellular) Antibody (#ANR-155), (1:300), followed by goat anti-rabbit-AlexaFluor-488. A. ADAM23 immunoreactivity (green) appears in Purkinje cells (arrows). B. Pre-incubation of the antibody with ADAM23 (extracellular) Blocking Peptide (BLP-NR155), suppressed staining. Cell nuclei are stained with DAPI (blue).
Expression of ADAM23 in mouse cerebellum.Immunohistochemical staining of perfusion-fixed frozen mouse brain sections with Anti-ADAM23 (extracellular) Antibody (#ANR-155), (1:300), followed by goat anti-rabbit-AlexaFluor-488. A. ADAM23 immunoreactivity (green) appears in Purkinje cells (arrows). B. Pre-incubation of the antibody with ADAM23 (extracellular) Blocking Peptide (BLP-NR155), suppressed staining. Cell nuclei are stained with DAPI (blue).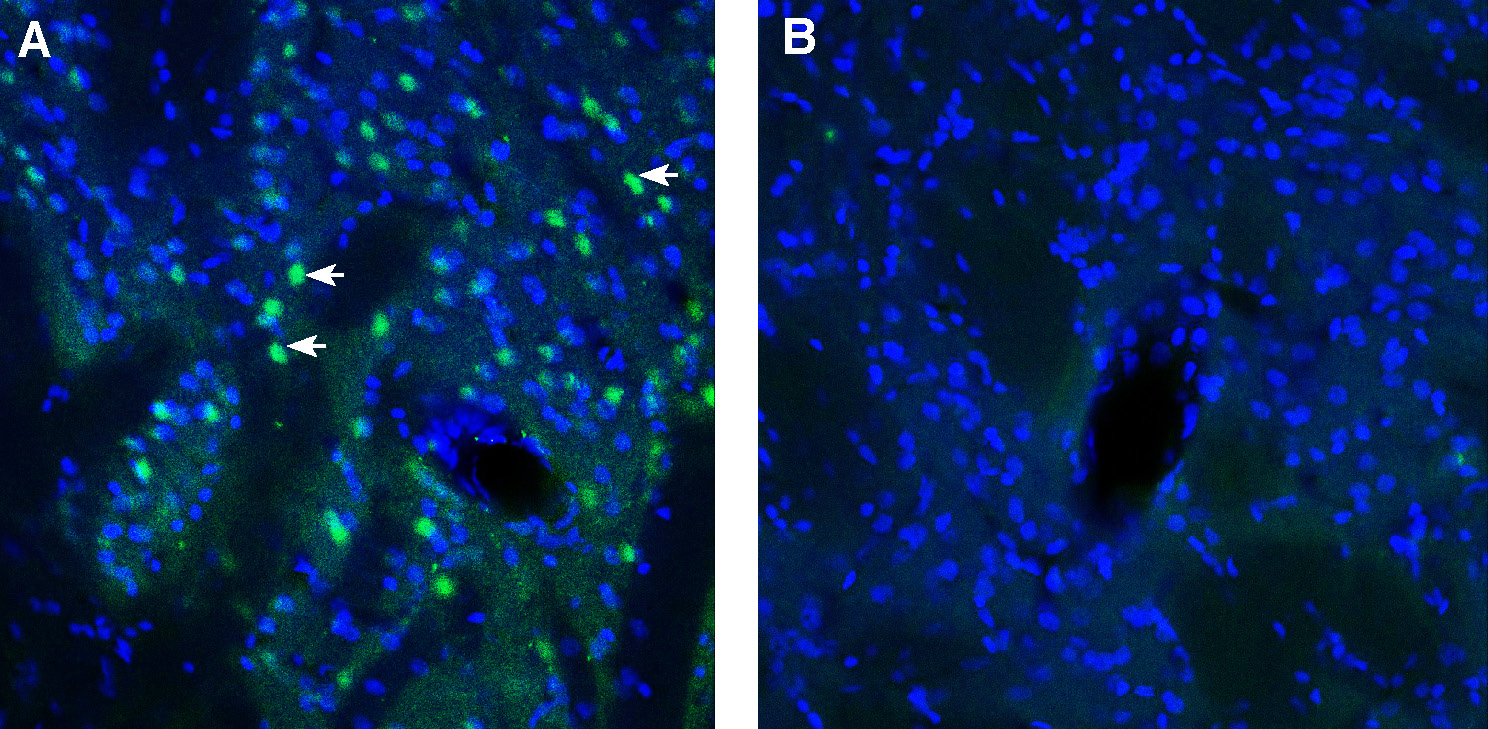 Expression of ADAM23 in rat striatum.Immunohistochemical staining of perfusion-fixed frozen mouse brain sections with Anti-ADAM23 (extracellular) Antibody (#ANR-155), (1:300), followed by goat anti-rabbit-AlexaFluor-488. A. ADAM23 immunoreactivity (green) appears in striatal cells (arrows). B. Pre-incubation of the antibody with ADAM23 (extracellular) Blocking Peptide (BLP-NR155), suppressed staining. Cell nuclei are stained with DAPI (blue).
Expression of ADAM23 in rat striatum.Immunohistochemical staining of perfusion-fixed frozen mouse brain sections with Anti-ADAM23 (extracellular) Antibody (#ANR-155), (1:300), followed by goat anti-rabbit-AlexaFluor-488. A. ADAM23 immunoreactivity (green) appears in striatal cells (arrows). B. Pre-incubation of the antibody with ADAM23 (extracellular) Blocking Peptide (BLP-NR155), suppressed staining. Cell nuclei are stained with DAPI (blue).
- Souza ILM, et al. (2021) Exp Cell Res. 398, 2.
- Camodeca C, et al. (2019) Curr Med Chem. 26, 15.
- Borgonovo ZLM, et al. (2018) Neuroscience. 384, 165-177.
- Lancaster, Eunjoo et al. (2019) Neuroscience letters 704, 159-163.
- Owuor, Katherine et al. (2009) Molecular and cellular neurosciences vol. 42, 4.
- Verbisck NV, et al. (2009) Cancer Res. 69, 13.
A Disintegrin And Metalloprotease 23 (ADAM23) is a member of the ADAMs family of transmembrane proteins, mostly expressed in the nervous system, and involved in traffic and stabilization of Kv1-potassium channels, synaptic transmission, neurite outgrowth, neuronal morphology and cell adhesion. ADAM23 has been linked to human pathological conditions, such as epilepsy, cancer metastasis and cardiomyopathy.[1]
The ADAMs are a family of transmembrane and secreted proteins of approximately 750 amino acids in length. They differ in structural organization, function and localization. Their domains, catalytic or non-catalytic, determine their substrate recognition and protease activity. Some ADAMs show sheddase activity, which modulates functional proteins such as TNFs, growth factors, cytokines, and adhesion proteins. [2]
ADAM23 is a transmembrane type I glycoprotein. Since its metalloprotease domain is inactive, it appears to function mainly through the disintegrin domain. ADAM23 has been shown to regulate mechanisms in epilepsy, inflammation and tumor establishment through interaction with different members of integrin receptors.[3]
ADAM23 is thought to form a trans-synaptic complex with ADAM22 and LGI1. ADAM23 binds Kv1.1/Kv1.4 potassium channels, ADAM22 binds post-synaptic AMPA receptors, and LGI1 align these pre- and post-synaptic elements. The interaction of LGI1 and the ADAM proteins appears to regulate excitability of CNS neurons. In addition to binding to LGI1, ADAM23 also regulates the amount of LGI1 molecules at the axon initial segment, by modulating the trafficking, export and expression of LGI1.[4] In knockout studies in mice, aberrant LGI1 regulation of neuronal morphology through ADAM23 protein resulted in Autosomal dominant lateral temporal epilepsy (ADLTE). Autoantibodies directed at LGI1 have been shown to disrupt the trans-synaptic complex and are associated with autoimmune encephalitis. ADAM23-null mice had aberrant dendrite morphology and exhibited seizures. Reduced ADAM23 expression lowered seizure threshold.[5]
ADAM23 has a specific interaction with alpha(v)beta(3) integrin mediated by the disintegrin domain. ADAM23 knockdown cells showed enhanced migration and adhesion to alpha(v)beta(3) integrin ligands. Expression of this integrin in the activated form has been shown to promote metastasis formation. This suggests a functional role of ADAM23 during metastatic progression by negatively modulating alpha(v)beta(3) integrin activation. It has been shown that the ADAM23 gene is frequently silenced in different types of tumors, including melanoma, glioma, prostate, ovary, and breast cancer.[6]
Application key:
Species reactivity key:
Anti-ADAM23 (extracellular) Antibody (#ANR-155) is a highly specific antibody directed against an extracellular epitope of the mouse protein. The antibody can be used in western blot, immunohistochemistry and flow cytometry applications. It has been designed to recognize ADAM23 from mouse, rat and human samples.

