Overview
- Peptide RTSDSRDHTRVDWKR(C), corresponding to amino acid residues 271-285 of rat GluR1 (Accession P19490). Extracellular, N-terminus.

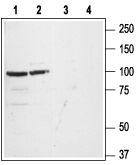 Western blot analysis of rat (lanes 1 and 3) and mouse (lanes 2 and 4) brain lysates:1,2. Anti-GluR1 (GluA1) (extracellular) Antibody (#AGC-004), (1:200).
Western blot analysis of rat (lanes 1 and 3) and mouse (lanes 2 and 4) brain lysates:1,2. Anti-GluR1 (GluA1) (extracellular) Antibody (#AGC-004), (1:200).
3,4. Anti-GluR1 (GluA1) (extracellular) Antibody, preincubated with GluR1/GluA1 (extracellular) Blocking Peptide (#BLP-GC004).
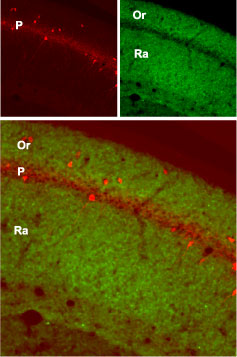 Expression of GluR1 in mouse hippocampusImmunohistochemical staining of mouse hippocampus with Anti-GluR1 (GluA1) (extracellular) Antibody (#AGC-004). GluR1 (green) is present in the stratum oriens (Or) and radiatum (Ra) but not in the pyramidal layer (P). Staining of the same section with mouse anti-parvalbumin (red) identifies the pyramidal layer.
Expression of GluR1 in mouse hippocampusImmunohistochemical staining of mouse hippocampus with Anti-GluR1 (GluA1) (extracellular) Antibody (#AGC-004). GluR1 (green) is present in the stratum oriens (Or) and radiatum (Ra) but not in the pyramidal layer (P). Staining of the same section with mouse anti-parvalbumin (red) identifies the pyramidal layer.
- Rat hippocampal neurons (16 μg/ml) (Verpelli, C. et al. (2011) J. Biol. Chem. 286, 34839.).
- Dingledine, R. et al. (1999) Pharmacol. Rev. 51, 7.
- Sheng, M. and Lee, S.H. (2001) Cell 105, 825.
- Song, I. and Huganir, R.L. (2002) Trends Neurosci. 25, 578.
AMPA receptors are members of the glutamate receptor family of ion channels that also include the NMDA and Kainate receptors. The three subfamilies are named after the original synthetic agonists that were identified as selective ligands of each family.
The α-amino-3-hydroxy-5-methyl-4-isoazolepropionic acid (AMPA) receptor subfamily includes four members AMPA1-AMPA4 that are also known as GluR1-GluR4 respectively.
The functional AMPA channel is believed to be a tetramer, with most neuronal AMPA receptors being actually heterotetramers composed of AMPA1 plus AMPA2 or AMPA2 plus AMPA3, although homotetramers can also be found.
AMPA receptors are permeable to cations Na+, K+ and Ca2+. The Ca2+ permeability is dependent on the presence of AMPA2: whenever this subunit is present, the channel will be impermeable to Ca2+. The Ca2+ permeability of the AMPA2 subunit is determined by the presence of an arginine (R) at a critical site in the pore loop instead of a glutamine (Q) present in the same site in the other AMPA subunits. A post-transcriptional process known as RNA editing determines the presence of this R. Since most AMPA2 subunits in the adult brain have undergone RNA editing and most AMPA receptors contain the AMPA2 subunit, most native AMPA receptors will be impermeable to Ca2+.
Gating of AMPA receptors by glutamate is extremely fast and therefore the AMPA receptors mediate most excitatory (depolarizing) currents in the brain during basal neuronal activity. The depolarization caused by the activation of post-synaptic AMPA receptors is necessary for the activation of NMDA receptors that will open only in the presence of both glutamate and a depolarized membrane.
Synaptic strength, defined as the level of post-synaptic depolarization, can be long term (hence the term long term potentiation, LTP) and therefore induce changes in signaling and protein synthesis in the activated neuron. These changes are associated with memory formation and learning.
Changes in synaptic strength are thought to involve rapid movement of the AMPA receptors in and out of the synapses and a great deal of effort has focused in understanding the mechanisms that govern AMPA receptor trafficking.
Application key:
Species reactivity key:
Anti-GluR1 (GluA1) (extracellular) Antibody (#AGC-004) is a highly specific antibody directed against an extracellular epitope of the rat ionotropic glutamate receptor 1. The antibody can be used in western blot, immunohistochemistry, immunocytochemistry, and live cell imaging applications. It has been designed to recognize GluR1 from human, mouse, and rat samples.
Applications
Citations
- Immunohistochemical staining of mouse brain sections. Tested in GLUA1-/- mice.
Egbenya, D.L. et al. (2018) Mol. Cell. Neurosci. 92, 93.
- Mouse brain lysate.
Hamada, S. et al. (2014) Eur. J. Neurosci. 40, 3136. - Rat cortical lysate (1:2500).
Banerjee, B. et al. (2013) Neurogastroenterol. Motil. 25, 973. - Mouse hippocampal lysate.
Ran, I. et al. (2013) J. Neurosci. 33, 1872. - Mouse brain lysate (1:2500).
Yu, Y.J. et al. (2013) Neurobiol. Learn. Mem. 103, 72. - Rat brain lysate.
Lin, H-C. et al. (2011) J. Neurosci. 31, 8851.
- Rat dissociated hippocampal neurons.
Hussain, S. and Davanger, S. (2015) PLoS ONE 10, e0140868. - Rat hippocampal neurons.
Okuno, H. et al. (2012) Cell 149, 886. - Rat hippocampal neurons (16 μg/ml).
Verpelli, C. et al. (2011) J. Biol. Chem. 286, 34839.
- Mouse brain sections. Also tested in GLUA1-/- mice.
Egbenya, D.L. et al. (2018) Mol. Cell. Neurosci. 92, 93. - Rat brain sections.
Ganea, D.A. et al. (2015) Neuropsychopharmacology 40, 2727.
- Rat hippocampal neurons (1:500).
Ambroziak, W. et al. (2018) Hippocampus 28, 707. - Transfected rat organotypic cultures.
Hamad, M.I. et al. (2011) Development 138, 4301. - Rat hippocampal neurons.
Oh, J.Y. et al. (2017) Neurosci. Lett. 649, 41. - Rat mixed spinal cord neurons.
Zhang, L. et al. (2017) eNeuro 4, e0175. - Mouse dopaminergic neurons.
Jang, M. et al. (2015) Sci. Rep. 5, 14773. - Rat spinal cord primary culture.
Hennekinne, L. et al. (2013) J. Neurosci. 33, 11432. - Rat hippocampal neurons (16 μg/ml).
Verpelli, C. et al. (2011) J. Biol. Chem. 286, 34839.
