Overview
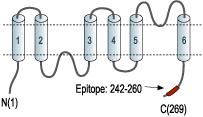
- Peptide (C)KVWTSGQVEEYDLDADDIN corresponding to amino acid residues 242-260 of human AQP1 (Accession P29972). Intracellular, C-terminus.
 Western blot analysis of rat kidney membranes:1. Anti-Aquaporin 1 Antibody (AQP-001), (1:400).
Western blot analysis of rat kidney membranes:1. Anti-Aquaporin 1 Antibody (AQP-001), (1:400).
2. Anti-Aquaporin 1 Antibody, preincubated with Aquaporin 1 Blocking Peptide (#BLP-QP001).
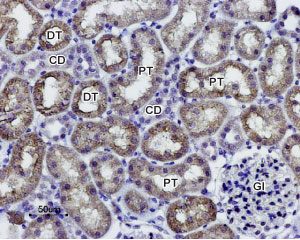 Expression of AQP1 in rat kidneyImmunohistochemical staining of paraffin-embedded rat kidney sections using Anti-Aquaporin 1 Antibody (#AQP-001), (1:100). AQP1 staining (brown) is present in both distal (DT) and proximal (PT) convoluted tubules in the renal cortex. Note that a weak staining is also present in collecting ducts (CD) while glomeruli (Gl) are negative. Hematoxilin is used as the counterstain.
Expression of AQP1 in rat kidneyImmunohistochemical staining of paraffin-embedded rat kidney sections using Anti-Aquaporin 1 Antibody (#AQP-001), (1:100). AQP1 staining (brown) is present in both distal (DT) and proximal (PT) convoluted tubules in the renal cortex. Note that a weak staining is also present in collecting ducts (CD) while glomeruli (Gl) are negative. Hematoxilin is used as the counterstain.- Mouse paraffin-embedded kidney sections (1:100) (Kim, J.I. et al. (2013) Am. J. Physiol. 304, F1283.).
- King, L.S. et al. (2004) Nat. Rev. Mol. Cell Biol. 5, 687.
- Ma, T. et al. (1998) J Biol Chem 273, 4296.
- Hu. J. and Verkman, A.S. (2006) FASEB J. 20, 1892.
Aquaporin 1 (AQP-1) belongs to a family of membrane proteins that allow passage of water and certain other solutes through biological membranes. The family is composed of 13 members (AQP-0 to AQP-12).
The aquaporins can be divided into two functional groups based on their permability characteristics: the aquaporins that are only permeated by water and the aquaglyceroporins that are permeated by water and other small solutes such as glycerol. AQP-1 together with AQP-2, AQP-4 and AQP-5 belongs to the first group1.
Little is known about the function of the two newest members, AQP-11 and AQP-12.
The proteins present a conserved structure of six transmembrane domains with intracellular N- and C-termini. The functional channel is a tetramer but each subunit has a separate pore and therefore the functional channel unit, contains four pores1.
AQP-1 is widely expressed in several organs with prominent expression found in kidney, lung, red blood cells intestine and brain. Studies with mice lacking AQP-1 show that the channel has a critical role in urine concentration2. In addition, AQP-1 expression in tumor cells was shown to contribute to enhanced tumor spread and metastatic potential3.
Application key:
Species reactivity key:
Anti-Aquaporin 1 Antibody (#AQP-001) is a highly specific antibody directed against an epitope of the human AQP1 channel. The antibody can be used in western blot and immunohistochemistry applications. It has been designed to recognize AQP1 from rat, mouse, and human samples.
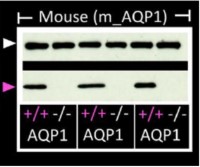
Knockout validation of Anti-Aquaporin 1 Antibody in mouse platelets.Western blot analysis of mouse platelets lysates using Anti-Aquaporin 1 Antibody (#AQP-001) (lower panel). AQP1 immunodetection is observe in platelets from wild type mice (+/+) but absent in platelets from AQP1-/- mice (-/-). GAPDH (upper panel) is used as the loading control.Adapted from Agbani, E.O. et al. (2018) JCI Insight 3, e99062. with permission of the American Society for Clinical Investigation.
Applications
Citations
 Expression of Aquaporin 1 in mouse kidneyImmunohistochemical staining of mouse kidney sections using Anti-Aquaporin 1 Antibody (#AQP-001). AQP1 staining (red) along the thin limb of Henle cells does not change following ischemia.
Expression of Aquaporin 1 in mouse kidneyImmunohistochemical staining of mouse kidney sections using Anti-Aquaporin 1 Antibody (#AQP-001). AQP1 staining (red) along the thin limb of Henle cells does not change following ischemia.
Adapted from Kim, J.I. et al. (2013) Am. J. Physiol. 304, F1283. with permission of The American Physiological Society.
- Mouse platelet lysate. Tested in AQP1-/- mice.
Agbani, E.O. et al. (2018) JCI Insight 3, e99062.
- Mouse platelet lysate. Tested in AQP1-/- mice.
Agbani, E.O. et al. (2018) JCI Insight 3, e99062. - Rat kidney lysate.
Chung, S. et al. (2019) Front. Physiol. 10, 271.
- Rat kidney sections.
Chung, S. et al. (2019) Front. Physiol. 10, 271. - Mouse kidney sections.
Kong, M.J. et al. (2019) Redox Biol. 20, 38. - Mouse kidney sections.
Han, S.J. et al. (2016) J. Am. Soc. Nephrol. 28, 1200. - Mouse paraffin-embedded kidney sections (1:100).
Kim, J.I. et al. (2013) Am. J. Physiol. 304, F1283.
- Ikarashi, N. et al. (2011) Am. J. Physiol. 301, G887.
- Kim, J. et al. (2011) Anat. Cell Biol. 44, 186.
- Kim, J. et al. (2010) Am. J. Physiol. 298, F1118.
- Park, J.W. et al. (2010) Am. J. Physiol. 298, F301.
- Satake, M. et al. (2010) Biol. Pharm. Bull. 33, 1965.
- Bae, E.H. et al. (2009) Nephrol. Dial. Transplant. 24, 2692.
- Kim, J. et al. (2009) Am. J. Physiol. 296, F622.
- Allory, Y. et al. (2008) Kidney Int. 73, 751.
- Lodewyck, D. et al. (2007) Arch Otolaryngol Head Neck Surg. 133, 557.
- Watson, K.J. et al. (2007) Chem. Senses. 32, 411.
- Lee-Kwon, W. et al. (2005) Am. J. Physiol. 288, C942.
- Mulder, J. et al. (2005) Am. J. Physiol. 288, R1417.
- Yeum, C.H. et al. (2003) Scand. J. Urol. Nephrol. 37, 99.
- Yoshino, J. et al. (2003) J. Am. Soc. Nephrol. 14, 3090.
- Kim, S.W. et al. (2001) J. Am. Soc. Nephrol. 12, 875.
- Kim, S.W. et al. (2001) J. Am. Soc. Nephrol. 12, 2019.
- Markert, J.M. et al. (2001) Physiol. Genomics 5, 21.
- Patil, R.V. et al. (2001) Am. J. Physiol. 281, C1139.
- Quigley, R. et al. (1998) J. Membr. Biol. 164, 177.
