Overview
- GST fusion protein with the sequence EYVFCPDVELKRRLKEAFSKAAQQTKGSYMEVEDNRSQVETEDLILKPGVVHVIDIDRGDEKKGKDSSGEVLSSV, corresponding to amino acid residues 249-323 of rat AQP4 (Accession P47863). Intracellular, C-terminus.

- Human AQP4 transfected in HEK-293 cells. (De Vidi, I. et al. (2011) Clin. Immunol. 138, 239.).
- Human AQP4 transfected in HEK-293 cells. (De Vidi, I. et al. (2011) Clin. Immunol. 138, 239.).
- The blocking peptide is not suitable for this application.
- King, L.S. et al. (2004) Nat. Rev. Mol. Cell Biol. 5, 687.
- Manley, G.T. et al. (2004) Neuroscience. 129, 983.
- Lennon, V.A. et al. (2005) J. Exp. Med. 202, 473.
Aquaporin 4 (AQP-4) belongs to a family of membrane proteins that allow passage of water and certain solutes through biological membranes. The family is composed of 13 members (AQP-0 to AQP-12).
The aquaporins can be divided into two functional groups based on their permeability characteristics: the aquaporins that are only permeated by water and the aquaglyceroporins that are permeated by water and other small solutes such as glycerol. AQP-4 together with AQP-1, AQP-2 and AQP-5 belong to the first group1. Little is known about the function of the two newest members, AQP-11 and AQP-12.
The proteins present a conserved structure of six transmembrane domains with intracellular N- and C-termini. The functional channel is a tetramer but each subunit has a separate pore and therefore the functional channel unit, contains four pores1.
AQP-4 is the major membrane water channel in the central nervous system. The channel is expressed in astrocyte foot processes in direct contact with capillary vessels in the brain suggesting a role in water transport under normal and pathological conditions. Indeed, transgenic mice lacking AQP-4 have reduced brain swelling and improved neurological outcome following water intoxication and focal cerebral ischemia. In contrast, brain swelling and clinical outcome are worse in AQP-4-null mice in models of vasogenic (fluid leak) edema caused by freeze-injury and brain tumor, probably due to impaired AQP-4-dependent brain water clearance2.
In addition, it has been recently shown that neuromyelitis optica (NMO), an inflammatory demyelinating disease that selectively affects optic nerves and spinal cord, is caused by the development of an autoantibody directed against AQP-43.
Application key:
Species reactivity key:
Alomone Labs is pleased to offer a highly specific antibody directed against an epitope located at the intracellular C-terminal domain of rat AQP4. Anti-Aquaporin 4 (AQP4) (249-323) Antibody (#AQP-004) can be used in western blot, immunocytochemical, immunohistochemical and indirect flow cytometry applications. It recognizes the Aquaporin 4 channel from rat, mouse and human samples.
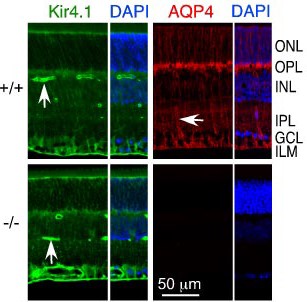
Knockout validation of Anti-Aquaporin 4 (AQP4) (249-323) Antibody in mouse retina.Immunohistochemical staining of mouse retina sections using Anti-Kir4.1 (KCNJ10) Antibody (#APC-035) and Anti-Aquaporin 4 (AQP4) (249-323) Antibody (#AQP-004). Kir4.1 staining (green) and AQP4 immunoreactivity (red) are polarized with greatest expression in inner retina containing the end foot processes. Lack of AQP4 staining in AQP4-/- mice (lower panels). DAPI (blue) is used to stain nuclei.Adaped from Ruiz-Ederra, J. et al. (2007) J. Biol. Chem. 282, 21866. with permission of the American Society for Biochemistry and Molecular Biology.
Applications
Citations
 AQP4 Serine phosphorylation is not required for plasma membrane trafficking.Immunocytochemical staining of C6 rat glial cells transfected with wild type and AQP4 mutants. Cells were stained with Anti-Aquaporin 4 (AQP4) (249-323) Antibody (#AQP-004). Compared to wild type, none of the Serine-to-Alanine AQP4 mutants are affected by their ability to reach the plasma membrane.
AQP4 Serine phosphorylation is not required for plasma membrane trafficking.Immunocytochemical staining of C6 rat glial cells transfected with wild type and AQP4 mutants. Cells were stained with Anti-Aquaporin 4 (AQP4) (249-323) Antibody (#AQP-004). Compared to wild type, none of the Serine-to-Alanine AQP4 mutants are affected by their ability to reach the plasma membrane.
Adapted from Assentoft, M. et al. (2014) with permission of American Physiological Society. Expression of Aquaporin 4 in mouse brain.Immunohistochemical staining of mouse brain sections using Anti-Aquaporin 4 (AQP4) (249-323) Antibody (#AQP-004). AQP4 staining (green) is detected in the cortex and hippocampus of normal mouse brain (NTG).
Expression of Aquaporin 4 in mouse brain.Immunohistochemical staining of mouse brain sections using Anti-Aquaporin 4 (AQP4) (249-323) Antibody (#AQP-004). AQP4 staining (green) is detected in the cortex and hippocampus of normal mouse brain (NTG).
Adapted from Zhang, X. et al. (2017) Front. Neurosci. 11, 212. with permission of Frontiers.
- Western blot analysis and immunohistochemical staining of mouse retina. Tested in AQP4-/- mice.
Ruiz-Ederra, J. et al. (2007) J. Biol. Chem. 282, 21866.
- Mouse primary astroglia cell lysate.
Rocchio, F. et al. (2019) Cell Death Dis. 10, 24. - Mouse retina lysate. Also tested in AQP4-/- mice.
Ruiz-Ederra, J. et al. (2007) J. Biol. Chem. 282, 21866.
- Mouse brain and spinal cord sections (1:200).
Guo, Y. et al. (2016) Mol. Neurobiol. 53, 3235. - Human thyroid sections.
Soelberg, K. et al. (2016) Neurol. Neuroimmunol. Neuroinflamm. 3, e252. - Mouse kidney.
Langaa, S. et al. (2012) Am. J. Physiol. 302, F1034. - Rat brain (1:200).
Jo, S.M. et al. (2011) Neurosci. Lett. 501, 25. - Immunohistochemistry of mouse retina sections. Also tested in AQP4-/- mice.
Ruiz-Ederra, J. et al. (2007) J. Biol. Chem. 282, 21866.
- Mouse primary astroglial cultures.
Rocchio, F. et al. (2019) Cell Death Dis. 10, 24. - Mouse muller cells.
Vacca, O. et al. (2016) Hum. Mol. Genet. 25, 3070. - C6 rat glia transfected cells.
Assentoft, M. et al. (2014) Am. J. Physiol. 307, C957. - Madin-Darby canine kidney cells (MDCK) (1:3000).
Arnspang, E.C. et al. (2013) PLoS ONE 8, e73977. - Xenopus oocytes (1:5000).
Assentoft, M. et al. (2013) Glia 61, 1101. - Human AQP4 transfected in HEK-293 cells.
De Vidi, I. et al. (2011) Clin. Immunol. 138, 239.
- Human AQP4 transfected in HEK-293 cells.
De Vidi, I. et al. (2011) Clin. Immunol. 138, 239.
- Toft-Bertelsen, T.L. et al. (2017) J. Physiol. 595, 3287.
- Zhang, X. et al. (2017) Front. Neurosci. 11, 212.
- Tham, D.K. et al. (2016) PLoS ONE 11, e0165439.
- Yde, J. et al. (2016) Front. Nutr. 3, 46.
- Kortenoeven, M.L.A. et al. (2013) J. Physiol. 591, 2205.
- Lien, C.F. et al. (2012) J. Biol. Chem. 287, 41374.
- Tham, D.K. and Moukhles, H. (2011) Am. J. Physiol. Renal Physiol. 301, F396.
- Merigo, F. et al. (2011) Dev. Neurobiol. 71, 854.
- Ikarashi, N, et al. (2011) Biol. Pharm. Bull. 34, 238.
- Noël, G. et al. (2010) PLoS One. 6, e17559.
- Satake, M. et al. (2010) Biol. Pharm. Bull. 33, 1965.
- Skjolding, A.D, et al. (2010) Cereb. Fluid Res. 7, 20.
- Toft-Hansen, H. et al. (2010) Glia 59, 166.
- Wang, Y-F. et al. (2009) J. Neurosci. 29, 1743.
- Zhang, H. and Verkman, A.S. (2008) Mol. Cell. Neurosci. 37, 1.
- Hibino, H. et al. (2007) Eur. J. Neurosci. 26, 2539.
- Ruiz-Ederra, J. et al. (2007) J. Biol. Chem. 282, 21866.
- Hibino, H. et al. (2004) J. Biol. Chem. 279, 44065.

