Overview
- Peptide (C)RLAYQGQDTPEVLMD, corresponding to amino acid residues 368 - 382 of human AXL (Accession P30530). Extracellular, N-term.
AXL (extracellular) Blocking Peptide (BLP-TR032)
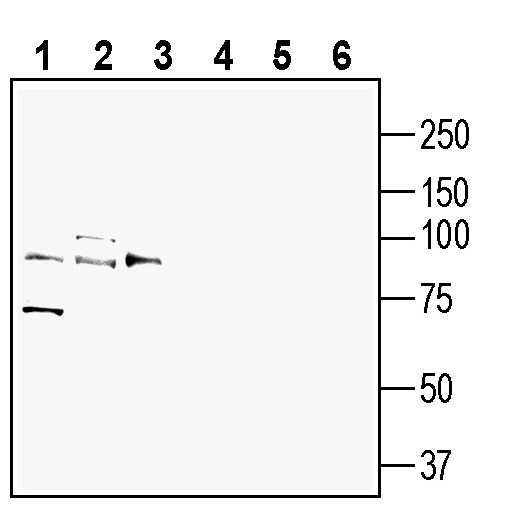 Western blot analysis of rat brain lysate (lanes 1 and 4), mouse brain membranes (lanes 2 and 5) and mouse lung lysate (lanes 3 and 6):1-3. Anti-AXL (extracellular) Antibody (#ATR-032), (1:200).
Western blot analysis of rat brain lysate (lanes 1 and 4), mouse brain membranes (lanes 2 and 5) and mouse lung lysate (lanes 3 and 6):1-3. Anti-AXL (extracellular) Antibody (#ATR-032), (1:200).
4-6. Anti-AXL (extracellular) Antibody, preincubated with AXL (extracellular) Blocking Peptide (BLP-TR032). Western blot analysis of human THP-1 monocytic leukemia cell line lysate (lanes 1 and 3) and human SW-403 colon adenocarcinoma cell line lysate (lanes 2 and 4):1-2. Anti-AXL (extracellular) Antibody (#ATR-032), (1:200).
Western blot analysis of human THP-1 monocytic leukemia cell line lysate (lanes 1 and 3) and human SW-403 colon adenocarcinoma cell line lysate (lanes 2 and 4):1-2. Anti-AXL (extracellular) Antibody (#ATR-032), (1:200).
3-4. Anti-AXL (extracellular) Antibody, preincubated with AXL (extracellular) Blocking Peptide (BLP-TR032).
AXL, also known as Tyrosine Kinase Receptor UFO and Tyro7, is a member of the receptor tyrosine kinase TAM family, that includes the receptors Tyro3, AXL and MERTK 1,2.
The TAM family receptors are widely expressed in normal cells and tissues, such as monocytes, platelets, endothelial cells, brain and heart, where they regulate cell survival, non-inflammatory clearance of apoptotic cells by phagocytic cells, natural killer cell differentiation, platelet aggregation, and more 1,2.
The TAM family receptors show structural similarities with other tyrosine kinase receptors, that is, an extracellular N-terminal domain containing two immunoglobulin-like and two fibronectin 3 domains, followed by a hydrophobic single pass transmembrane domain, and a cytoplasmic C-terminal tail containing a tyrosine kinase domain1,2.
Axl and the other TAM receptors can be activated via their ligands, growth arrest specific 6 protein (Gas6) and Protein S (Pros1), which are members of the family of vitamin K-dependent proteins 3.
Both AXL receptor and its high affinity ligand Gas6, are key regulators of immune cell activation, and have been shown to be expressed in cancer cells where they promote survival and invasion and contribute to resistance to various therapies 3,4,5. AXL expression is linked to increased risk of metastasis and poor survival in a variety of solid cancers including breast cancer, non-small cell lung carcinoma, ovarian cancer, and clear cell renal carcinoma 3,4. AXL activation in cancer cells and various stromal cells also results in tumor microenvironment deregulation, leading to modulation of angiogenesis, fibrosis, immune response and hypoxia 3,4.
Based on these findings, AXL has been identified as a critical therapeutic target for solid cancers, and indeed several therapeutic modalities including small molecules and monoclonal antibodies, are currently being developed 2, 4.
