Overview
- Peptide (C)DPAQGYKDHTLD, corresponding to amino acid residues 259-270 of mouse Bestrophin-2 (Accession Q8BGM5). 3rd extracellular loop.

 Western blot analysis of rat colon membranes:1. Anti-Bestrophin-2 (extracellular) Antibody (#ABC-002), (1:200).
Western blot analysis of rat colon membranes:1. Anti-Bestrophin-2 (extracellular) Antibody (#ABC-002), (1:200).
2. Anti-Bestrophin-2 (extracellular) Antibody, preincubated with Bestrophin-2 (extracellular) Blocking Peptide (#BLP-BC002). Western blot analysis of human colon cancer cell lines Colo-205 (lanes 1 and 4), human T-84 colonic adenocarcinoma cell line (lanes 2 and 5) and human colon cancer HT-29 cell line (lanes 3 and 6):1-3. Anti-Bestrophin-2 (extracellular) Antibody (#ABC-002), (1:200).
Western blot analysis of human colon cancer cell lines Colo-205 (lanes 1 and 4), human T-84 colonic adenocarcinoma cell line (lanes 2 and 5) and human colon cancer HT-29 cell line (lanes 3 and 6):1-3. Anti-Bestrophin-2 (extracellular) Antibody (#ABC-002), (1:200).
4-6. Anti-Bestrophin-2 (extracellular) Antibody, preincubated with Bestrophin-2 (extracellular) Blocking Peptide (#BLP-BC002).
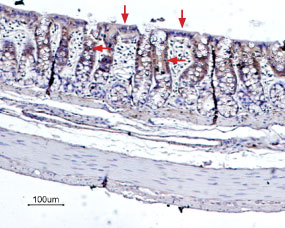 Expression of Bestrophin-2 in rat colonImmunohistochemical staining of rat colon paraffin embedded sections using Anti-Bestrophin-2 (extracellular) Antibody (#ABC-002), (1:100), (brown). BEST2 is expressed in the columnar absorptive cells of the colonic epithelium (arrows). Hematoxilin is used as the counterstain.
Expression of Bestrophin-2 in rat colonImmunohistochemical staining of rat colon paraffin embedded sections using Anti-Bestrophin-2 (extracellular) Antibody (#ABC-002), (1:100), (brown). BEST2 is expressed in the columnar absorptive cells of the colonic epithelium (arrows). Hematoxilin is used as the counterstain.
- Men, G. et al. (2004) Am. J. Ophtalmol. 137, 963.
- Petrukhin, K. et al. (1998) Nat. Genet. 19, 241.
- Hartzell, C.H. et al. (2008) Physiol. Rev. 88, 639.
- Kramer, F. et al. (2004) Cytogen. Genome Res. 105, 107.
- Stohr, H. et al. (2002) Eur. J. Hum. Genet. 10, 281.
- Park, H. et al. (2009) J. Neurosci. 29, 13063.
- Pifferi, S. et al. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12929.
- Tsunenari, T. et al. (2003) J. Biol. Chem. 278, 41114.
- Milenkovic, V.M. et al. (2007) J. Biol. Chem. 282, 1313.
- Xia, Q. et al. (2008) J. Gen. Physiol. 132, 681.
- Chien, L.T. et al. (2006) J. Gen. Physiol. 128, 247.
- Tsunenari, T. et al. (2006) J. Gen. Physiol. 127, 749.
- Qu, Z. et al. (2003) J. Biol. Chem. 278, 49563.
Mammalian Cl- channels can be broadly classified into four different families: voltage-dependent Cl- channels (CLCs), the cystic fibrosis transmembrane conductance regulator (CFTR), ligand-gated Cl- channels (γ-aminobutyric acid (GABA)) and glycine channels) and Ca2+-activated Cl- channels (Bestrophin and Anoctamin channels).
Bestrophins were first found by genetic linkage of human-Bestrophin-1 (hBest1) to a juvenile form of macular degeneration called Best vitelliform macular dystrophy1,2. To date Bestrophin 1-4 have been identified, although Bestrophin-3 and Bestrophin-4 have been observed only at the RNA level3. In addition, splice variants of some of these Ca2+-activated Cl- channels (CaCCs) have also been detected2,4,5. CaCCs are known to be involved in the regulation of olfaction, taste, phototransduction, and excitability in the nervous system. However, the molecular identity and functional role of CaCC in the brain have not been well established6. Bestrophin-2 is prominently expressed in colon and testes. Recently, Bestrophin-2 has also been found to be expressed in olfactory sensory neurons (OSNs)7.
Two different topologies for Bestrophin-1 have been proposed. The first structure proposes that six hydrophobic domains span the membrane8, while the second suggests that there are only four membrane-spanning domains9. The same structural controversy applies to Bestrophin-2. Bestrophin-1 and Bestrophin-2 (as well as Bestrophin-4 by heterologous expression) are activated by intracellular Ca2+ 10-12. Ca2+-dependent activation of Bestrophin-2 was also demonstrated in Xenopus13.
Application key:
Species reactivity key:
Alomone Labs is pleased to offer a highly specific antibody directed against an epitope located at the 3rd extracellular loop of mouse Bestrophin-2. Anti-Bestrophin-2 (extracellular) Antibody (#ABC-002) can be used in western blot and immunohistochemical applications, and has been designed to recognize bestrophin-2 channel from mouse, rat and human samples.
Applications
Citations
- Mouse colon epithelia lysate.
Rottgen, T.S. et al. (2018) Am. J. Physiol. 315, C10.
