Overview
- Peptide (C)TTKINMDDLQPSENEDKS, corresponding to amino acid residues 848-865 of rat CaV1.2 (Accession P22002). Intracellular loop between domains II and III.
- Rat brain membrane (1:200).
 Western blot analysis of rat brain membrane:1. Guinea pig Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003-GP), (1:200).
Western blot analysis of rat brain membrane:1. Guinea pig Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003-GP), (1:200).
2. Guinea pig Anti-CaV1.2 (CACNA1C) Antibody, preincubated with Cav1.2/CACNA1C Blocking Peptide (#BLP-CC003).- Following a broad screen of secondary antibodies, the following was used for this application: #106-035-006 (Jackson ImmunoResearch).
- Rat heart and pancreas and cerebellum (1:100). Following a broad screen of secondary antibodies, the following was used for this application: #106-485-006 (Jackson ImmunoResearch).
Voltage-gated Ca2+ channels (CaV), enable the passage of Ca2+ ions in a voltage dependent manner. These heteromeric entities are formed in part by the pore-forming α1 subunit which determines the biophysical and pharmacological properties of the channel1.
L-type Ca2+ channels make up one of three voltage-gated Ca2+ channel families. Four different α1 isoforms (CaV1.1 to CaV1.4) belong to the L-type subfamily. Structurally, each α1 subunit has four homologous domains (I-IV) and each domain has a six transmembrane section. Like many other voltage-gated channels, L-type Ca2+ channels have auxiliary subunits which are responsible for modulating the surface expression and properties of the channels2-5.
CaV1.1 is mostly expressed in the skeletal muscle, while CaV1.4 is mainly detected in the retina. The expression of both CaV1.2 and CaV1.3 is more extensive and includes neurons, heart, smooth muscle, inner ear, retina and pancreas6. L-type Ca2+ channels are involved in and modulate a variety of physiological functions such as muscle contraction, hormone secretion, neuronal excitability and gene expression5.
CaV1.2 undergoes various post-translational modifications. For example, it can undergo proteolytic cleavage at its C-terminal. This cleavage has been shown to take place in neurons following the activation of NMDA receptors5,7 and in the heart5,8,9. The cleaved moiety can still interact with the channel and its general purpose is to modulate channel activity5. Other postranslation modifications of the channel include phosphorylation of CaV1.2 by a number of kinases such as PKA, PKC, Src and CaMKII5. In addition, it is not surprising that phosphatases also regulate channel activity, as they are required to antagonize the activity of the various kinases known to phosphorylate CaV1.2 5.
The fact that CaV1.2 plays a prominent role in proper cardiac function has prompted endless studies regarding its regulation. Such studies have concluded that dysregulation of the channel leads to anomalies in heart contraction and thus heart failure5. Likewise, CaV1.2 defects have been detected in autism and bipolar disorder10.
Application key:
Species reactivity key:
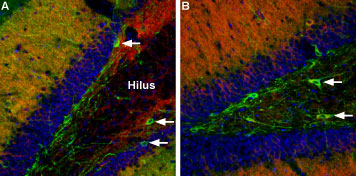
Multiplex staining of CaV1.2 and GABA(A) α1 Receptor in rat and mouse hippocampusImmunohistochemical staining of mouse and rat hippocampal dentate gyrus using Guinea pig Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003-GP) and Anti-GABA(A) α1 Receptor (extracellular)-ATTO Fluor-488 Antibody (#AGA-001-AG) in the same section. Both CaV1.2 (red) and GABA(A) α1 Receptor (green) are detected in neuron-shaped cells (arrows). Staining suggests partial colocalization between CaV1.2 and GABA(A) α1 Receptor in a sub-population of dentate gyrus neurons. A. Mouse hippocampus. B. Rat hippocampus.
