Overview
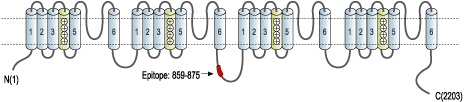
- Peptide (C)DNKVTIDDYQEEAEDKD, corresponding to amino acid residues 859-875 of rat CaV1.3 (Accession P27732). Intracellular loop between domains II and III.
 Western blot analysis of mouse brain (lanes 1 and 4), rat brain (lanes 2 and 5) and human brain neuroblastoma cells (SH-SY5Y) (lanes 3 and 6):
Western blot analysis of mouse brain (lanes 1 and 4), rat brain (lanes 2 and 5) and human brain neuroblastoma cells (SH-SY5Y) (lanes 3 and 6):1-3. Guinea pig Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005-GP), (1:200).
4-6. Guinea pig Anti-CaV1.3 (CACNA1D) Antibody, preincubated with Cav1.3/CACNA1D Blocking Peptide (#BLP-CC005).
Following a broad screen of secondary antibodies, the following were used for this application:
Western blot analysis:
#106-035-006 (Jackson ImmunoResearch)
#A7289 (Sigma)
 Expression of CaV1.3 in rat DRGsImmunohistochemical staining of rat dorsal root ganglion (DRG) using Guinea pig Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005-GP), (1:200). A. CaV1.3 staining (red) is detected in some DRG cells (arrows). B. Nuclei staining using DAPI as the counterstain. C. Merged image of panels A and B.
Expression of CaV1.3 in rat DRGsImmunohistochemical staining of rat dorsal root ganglion (DRG) using Guinea pig Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005-GP), (1:200). A. CaV1.3 staining (red) is detected in some DRG cells (arrows). B. Nuclei staining using DAPI as the counterstain. C. Merged image of panels A and B.
- Striessnig, J. et al. (2014) Wiley Interdiscip. Rev. Membr. Transp. Signal. 3, 15.
- Striessnig, J. et al. (2010) Pflugers Arch. 460, 361.
The CaV1.3 channel belongs to the L-type family of Ca2+ channels. There are currently four known members in this family. L-type channels provide the main influx pathway for Ca2+, an essential, versatile and universal intracellular messenger.
CaV1.3, as well as the other members of its family, is comprised of an α1-subunit that serves as the voltage sensitive pore. This subunit is comprised of transmembrane segments S1–S4 and a pore-forming region comprised of S5 and S6 segments together with their connecting linker, which contains helical regions contributing to the formation of the selectivity filter. The α1-subunit associates with other subunits to form hetero-oligomeric complexes. β-subunits are tightly associated at the cytoplasmic face of α1, whereas α2δ subunits are GPI-anchored to the plasma membrane and interact with the extracellular domains of α1. The long cytoplasmic C-terminal region of the α1 subunits serves as an important modulatory domain and is a target of numerous protein–protein interactions.
CaV1.3 is expressed in many tissues such as the sinoatrial node, heart atria, neurons, chromaffin cells and pancreatic islets. Currently available calcium channel blocking therapeutic agents block CaV1.2 and CaV1.3 making it difficult to distinguish between the two. To this day there are no known human diseases resulting from mutations in the CACNA1D gene encoding the CaV1.3 α1 subunit. This could be due to the fact that loss-of-function mutations cause no phenotype in the heterozygous state (in mice) but are lethal in the homozygous state1,2.
Application key:
Species reactivity key:
Alomone Labs is pleased to offer a highly specific antibody directed against an epitope of rat CaV1.3 channel. Guinea pig Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005-GP, formerly AGP-061), raised in guinea pigs, can be used in western blot analysis, and has been designed to recognize CaV1.3 from mouse, rat, and human samples. The antigen used to immunize guinea pigs is the same as Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005) raised in rabbit. Our line of guinea pig antibodies enables more flexibility with our products such as multiplex staining studies, immunoprecipitation, etc.
