Overview
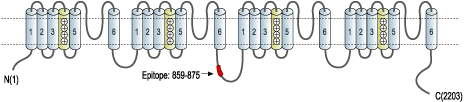
- Peptide (C)DNKVTIDDYQEEAEDKD, corresponding to amino acid residues 859-875 of rat CaV1.3 (Accession P27732). Intracellular loop between domains II and III.
 Western blot analysis of rat brain membranes:1. Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), (1:200).
Western blot analysis of rat brain membranes:1. Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), (1:200).
2. Anti-CaV1.3 (CACNA1D) Antibody, preincubated with Cav1.3/CACNA1D Blocking Peptide (#BLP-CC005).- Human SAN tissue (Le Scouarnec, S. et al. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15617.).
- Rat cerebral cortex, thalamus, cerebellum, hippocampus, and spinal cord lysates (Kim, S. et al. (2007) J. Biol. Chem. 282, 32877.).
 Expression of CaV1.3 in rat DRGImmunohistochemical staining of adult rat dorsal root ganglion (DRG) with Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005). Staining appears in clusters of cells (green) but not in axons. Staining of neurofilament 200 demonstrates partial overlap with CaV1.3 in neuronal staining but not in axons (red).
Expression of CaV1.3 in rat DRGImmunohistochemical staining of adult rat dorsal root ganglion (DRG) with Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005). Staining appears in clusters of cells (green) but not in axons. Staining of neurofilament 200 demonstrates partial overlap with CaV1.3 in neuronal staining but not in axons (red).
- Mouse SAN cells (Le Scouarnec, S. et al. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15617.).
- Catterall, W.A. et al. (2003) Pharmacol. Rev. 55, 579.
- IUPHAR
- Mangoni, M.E. et al (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5543.
- Zhang, Z. et al. (2005) Circulation 112, 1936.
All L-type calcium channels are encoded by one of the CaV1 channel genes. These channels play a major role as a Ca2+ entry pathway in skeletal cardiac and smooth muscles as well as in neurons, endocrine cells and possibly in non-excitable cells such as hematopoetic and epithelial cells. All CaV1 channels are influenced by dihydropyridines (DHP) and are also referred to as DHP receptors. While the CaV1.1 and CaV1.4 isoforms are expressed in restricted tissues (skeletal muscle and retina, respectively), the expression of CaV1.2 is ubiquitous.1,2
The CaV1.3 channels are also expressed, as are other L-type channels, in neurons and neuroendocrine cells. However, accumulated data has shown the expression of CaV1.3 in heart and suggests that it plays a major role in the generation of cardiac pacemaker activity.3,4
Several peptidyl toxins have been described that are specific L-type channel blockers. These include the Mamba toxins Calcicludine (#SPC-650), Calciseptine (#C-500) and FS-2 (#F-700). So far no selective blocker for one of the CaV1 isoforms has been described.
Application key:
Species reactivity key:
Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunoprecipitation, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize CaV1.3 from mouse, rat, and human samples.
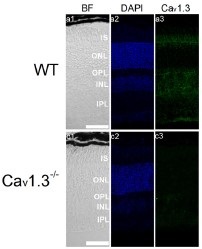
Knockout validation of Anti-CaV1.3 (CACNA1D) Antibody in mouse eye sections.Immunohistochemical staining of mouse eye sections using Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005). CaV1.3 immunoreactivity (green) is detected in major retinal layers (upper panel). CaV1.3 is not detected in CaV1.3-/- mice.Adapted from Shi, L. et al. (2017) Front. Cell. Neurosci. 11, 232. with permission of Frontiers.
Applications
Citations
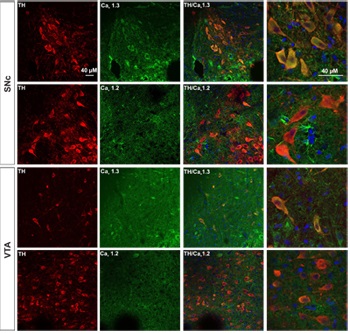 Expression of CaV1.3 in DA neurons from SNc and VTA.Immunohistochemical staining of rat brain sections using Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003) or Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), shows that CaV1.3 channel (green) is detected in SNc and VTA regions while little or no CaV1.2 is expressed. CaV1.3 co-localizes with TH (red) in the soma as well as in the proximal dendrites of SNc and VTA DA neurons.
Expression of CaV1.3 in DA neurons from SNc and VTA.Immunohistochemical staining of rat brain sections using Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003) or Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), shows that CaV1.3 channel (green) is detected in SNc and VTA regions while little or no CaV1.2 is expressed. CaV1.3 co-localizes with TH (red) in the soma as well as in the proximal dendrites of SNc and VTA DA neurons.
Adapted from Philippart, F. et al. with permission of the Society for Neuroscience.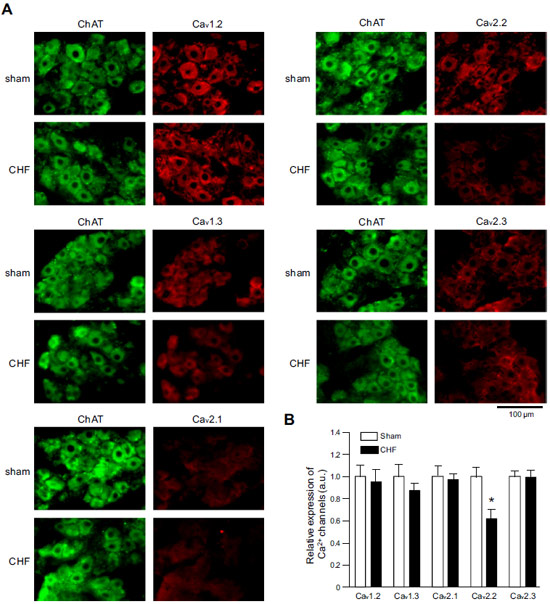 Expression of CaV α subunits in rat ICG.Immunohistochemical staining of rat intracardiac ganglia (ICG) using Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003), Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), Anti-CACNA1B (CaV2.2) Antibody (#ACC-002) and Anti-CaV2.3 (CACNA1E) Antibody (#ACC-006). All CaV subtypes are expressed in sham treated rats but N-type CaV channel levels are decreased in CHF rats.
Expression of CaV α subunits in rat ICG.Immunohistochemical staining of rat intracardiac ganglia (ICG) using Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003), Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), Anti-CACNA1B (CaV2.2) Antibody (#ACC-002) and Anti-CaV2.3 (CACNA1E) Antibody (#ACC-006). All CaV subtypes are expressed in sham treated rats but N-type CaV channel levels are decreased in CHF rats.
Adapted from Tu, H. et al. (2014) with permission of the American Physiological Society. Expression of CaV1.3 in mouse cochlea.Immunohistochemical staining of mouse cochlear sections using Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005). CaV1.3 staining (red) is detected in both apical (upper panels) and basal (lower panels) regions of the cochlea. Neurons were labeled with Tuj1 (green) and nuclei were stained with DAPI (blue). Scale bar, 20 μm.
Expression of CaV1.3 in mouse cochlea.Immunohistochemical staining of mouse cochlear sections using Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005). CaV1.3 staining (red) is detected in both apical (upper panels) and basal (lower panels) regions of the cochlea. Neurons were labeled with Tuj1 (green) and nuclei were stained with DAPI (blue). Scale bar, 20 μm.
Adapted from Lv, P. et al. (2014) with permission of the Society for Neuroscience.
- Immunohistochemical staining of mouse retina sections. Tested in CaV1.3-/- mice.
Shi, L. et al. (2017) Front. Cell. Neurosci. 11, 232.
- Human SH-SY5Y cell lysate and mouse brain lysate.
Sun, Y. et al. (2017) J. Neurosci. 37, 3364. - Mouse cochlea lysate (1:200).
Vincent, P.F. et al. (2017) J. Neurosci. 37, 2960. - Mouse astrocyte lysate (1:1000).
Cheli, V.T. et al. (2016) Glia 64, 1396. - Mouse heart lysate.
Walton, R.D. et al. (2016) J. Gerontol. A Biol. Sci. Med. Sci. 71, 1005. - Mouse eye lysate.
Grabner, C.P. et al. (2015) J. Neurosci. 35, 13133. - Rat retinae lysate (1:300).
Grimes, W.N. et al. (2015) J. Neurophysiol. 114, 341. - Rat heart lysates (1:1000).
Liao, P. et al. (2015) J. Biol. Chem. 290, 9262. - Rat brain lysates (1:200).
N’Gouemo, P. et al. (2015) Int. J. Neuropsychopharmacol. 18, pyu123. - Avian retinal lysates.
Huang, C.C. et al. (2013) PLoS ONE 8, e73315. - Chick retina lysate.
Ko, M.L. et al. (2013) J. Neurochem. 127, 314. - Mouse mesenteric and pulmonary arteries (1:200).
Ko, E.A. et al. (2013) Am. J. Physiol. 304, C1042. - Rat primary submucosa cells.
Rehn, M. et al. (2013) Cell Tissue Res. 353, 355. - Human SAN tissue.
Le Scouarnec, S. et al. (2008) Proc. Natl. Acad. Sci. 105, 15617.
- Rat retinae lysate (1-2 µg / 500 µg protein).
Grimes, W.N. et al. (2015) J. Neurophysiol. 114, 341. - Transfected HeK 293 cells.
Reichhart, N. et al. (2015) Cell. Signal. 27, 2231. - Rat cerebral cortex, thalamus, cerebellum, hippocampus, and spinal cord lysates.
Kim, S. et al. (2007) J. Biol. Chem. 282, 32877.
- Mouse retina sections. Also tested in CaV1.3-/- mice.
Shi, L. et al. (2017) Front. Cell. Neurosci. 11, 232. - Mouse cochlea sections.
Vincent, P.F. et al. (2017) J. Neurosci. 37, 2960. - Mouse inner ear sections (1:75).
Ohn, T.L. et al. (2016) Proc. Natl. Acad. Sci. U.S.A. 113, E4716. - Rat brain sections (1:100).
Philippart, F. et al. (2016) J. Neurosci. 36, 7234. - Mouse brain sections (1:200).
Blosa, M. et al. (2015) J. Physiol. 593, 4341. - Mouse eye sections.
Grabner, C.P. et al. (2015) J. Neurosci. 35, 13133. - Mouse cochlear sections (1:50).
Jung, S. et al. (2015) Proc. Natl. Acad. Sci. U.S.A. 112, E3141. - Mouse brain sections (1:200).
Kim, Y.S. et al. (2015) Eur. J. Neurosci. 42, 2467. - Mouse eye sections (1:100).
Reichhart, N. et al. (2015) Cell. Signal. 27, 2231. - Mouse cochlear sections.
Lv, P. et al. (2014) J. Neurosci. 34, 7383. - Mouse cochlea.
Pirone, A. et al. (2014) J. Neurosci. 34, 434. - Rat intracardiac ganglia and stellate ganglia neurons.
Tu, H. et al. (2014) Am. J. Physiol. 306, C132. - Human and mouse fetal lung sections (1:50 - 1:100).
Brennan, S.C. et al. (2013) PLoS ONE 8, e80294. - Human adrenal cortex sections (1:100-1:250).
Scholl, U.I. et al. (2013) Nat. Genet. 45, 1050. - Mouse apical cochlear turns (1:75).
Wong, A.B. et al. (2013) J. Neurosci. 33, 10661.
- Mouse astrocytes (1:200).
Cheli, V.T. et al. (2016) Glia 64, 1396. - Mouse spinal ganglion neurons (SGNs) (1:100-1:500).
Lv, P. et al. (2014) J. Neurosci. 34, 7383. - Rat pinealocytes.
Mizutani, H. et al. (2014) Am. J. Physiol. 306, C1008. - Avian cultured retinas (1:100).
Huang, C.C. et al. (2013) PLoS ONE 8, e73315. - Rat primary submucosa cells (1:50).
Rehn, M. et al. (2013) Cell Tissue Res. 353, 355. - Rat SCG and hippocampal pyramidal neurons (1:1000).
Wheeler, D.G. et al. (2012) Cell 149, 1112. - Mouse SAN cells.
Le Scouarnec, S. et al. (2008) Proc. Natl. Acad. Sci. 105, 15617.
- Jing, Z. et al. (2013) J. Neurosci. 33, 4456.
- Johnson, S.L. et al. (2013) Proc. Natl. Acad. Sci. U.S.A. 110, 8720.
- Reinbothe, T.M. et al. (2013) Diabetologia 56, 340.
- Xie, L. et al. (2013) Traffic 14, 428.
- Christel, C.J. et al. (2012) J. Physiol. 590, 6327.
- Lv, P. et al. (2012) J. Neurosci. 32, 16314.
- Walter, H.J. et al. (2000) J. Biol. Chem. 275, 25717.
- Bae, I.H. et al. (1999) Korean J. Biol. Sci. 3, 53.
- Hernandez, M.E. et al. (1999) Neuroendocrinology 70, 31.
- Jiang, Z. et al. (1999) Eur. J. Neurosci. 11, 3481.
- Lopez, I. et al. (1999) Neuroscience 92, 773.
- Serrano, C.J. et al. (1999) FEBS Letters 462, 171.
- Yang, S.N. et al. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 10164.
