Overview
- Peptide (C)DRATGEHASVHEYPGE, corresponding to amino acid residues 456-471 of rat CACNB1 (Accession P54283). Intracellular, adjacent to the C-terminus.
- Rat and mouse brain membranes and rat cortex lysate (1:200-1:1000).
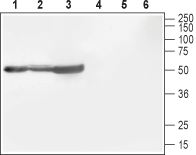 Western blot analysis of rat brain (lanes 1 and 4), mouse brain (lanes 2 and 5) and rat cortex (lane 3 and 6):1-3. Anti-CACNB1 Antibody (#ACC-106), (1:800).
Western blot analysis of rat brain (lanes 1 and 4), mouse brain (lanes 2 and 5) and rat cortex (lane 3 and 6):1-3. Anti-CACNB1 Antibody (#ACC-106), (1:800).
4-6. Anti-CACNB1 Antibody, preincubated with CACNB1 Blocking Peptide (#BLP-CC106).
- Rat brain.
Voltage-gated Ca2+ (CaV) channels are ubiquitously expressed and function as Ca2+ conducting pores in the plasma membrane1. Based on their electrophysiological and pharmacological properties, CaV channels have traditionally been classified into L, T, N, P, Q, and R types2. L-type Ca2+ channels are heteromultimers composed of four independently encoded proteins, the pore-forming α1 subunit, which triggers Ca2+ flow across the membrane, and the subunits α2δ, γ, and β3.
CaVβ subunits play critical roles in membrane trafficking of the channel complex and regulation of voltage-dependent gating. The CaVβ subunit binds to the endoplasmic reticulum (ER) retention signal in the I-II loop of the α1-subunit, which allows channels to traffic to the surface membrane4. Furthermore, CaVβ subunits not only allow for membrane trafficking of the channel complex, they also can play a role in determining the subcellular localization of channels on the surface membrane5. There are four distinct CaVβ subunits CaVβ1, CaVβ2, CaVβ3 and CaVβ46.
Three splice variants exist for the β1 subunit: β1a, β1b and β1c. β1a is known to be expressed in skeletal muscle and brain, but not in smooth muscle or heart. β1a appears to be important for the functional expression of the α1 subunit in skeletal muscle7. β1b was identified by cloning in rat brain, heart and hippocampus, and differs from β1a by having a deletion of ~50 amino acids at residue 209, and having a 120-residue C-terminal elongation. β1c was cloned from human heart and hippocampus and has the same deletion as β1b, but lacks the C-terminal extension.
