Overview
- Peptide (C)PSSPERAPGREGPYGRE, corresponding to amino acid residues 865-881 of rat CACNA1A (Accession P54282). Intracellular loop between domains II and III.

 Western blot analysis of rat brain membranes:1. Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), (1:200).
Western blot analysis of rat brain membranes:1. Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), (1:200).
2. Anti-CACNA1A (CaV2.1) Antibody, preincubated with CACNA1A/Cav2.1 Blocking Peptide (#BLP-CC001).- Human red blood cells (Andrews, D.A. et al. (2002) Blood 100, 3392.).
 Expression of CACNA1A in mouse cerebellumImmunohistochemical staining of mouse cerebellum with Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), (1:100). A. CACNA1A channel (red) appears in Purkinje cells (horizontal arrows) and is distributed diffusely in the molecular layer (Mol) including in astrocytic fibers (vertical arrows). B. Staining of astrocytic fibers with glial fibrillary acidic protein in the section demonstrates the location of astrocytic fibers in the molecular layer. C. Merged image of panels A and B.
Expression of CACNA1A in mouse cerebellumImmunohistochemical staining of mouse cerebellum with Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), (1:100). A. CACNA1A channel (red) appears in Purkinje cells (horizontal arrows) and is distributed diffusely in the molecular layer (Mol) including in astrocytic fibers (vertical arrows). B. Staining of astrocytic fibers with glial fibrillary acidic protein in the section demonstrates the location of astrocytic fibers in the molecular layer. C. Merged image of panels A and B.
- Mouse sperm cells (Serrano, C.J. et al. (1999) FEBS Lett. 462, 171.).
- Tsunemi, T. et al. (2002) J. Biol. Chem. 277, 7214.
- Scheuber, A. et al. (2004) J. Neurosci. 24,10402.
- Ophoff, R.A. et al (1996) Cell 87, 543.
- Ishikawa, K. et al. (1997) Am. J. Hum. Genet. 61, 336.
- Tottene, A. et al. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 13284.
- Mintz, I.M. et al. (1992) Nature 355, 827.
- Moreno, H. et al. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 14042.
- Bourinet, E. et al. (1999) Nat. Neurosci. 2, 407.
Voltage-dependent Ca2+ channels (CaV channels) are pivotal players in many physiological roles such as secretion, contraction, migration and excitation.1
The voltage-dependent calcium channels are composed of several subunits; α1, β, α2δ and γ. CaV channels were originally divided into six physiological types: L-, N-, P-, Q-, R-, and T-type.
The CaV2.1 (formally named α1A) makes up the α1 poreforming subunit in P/Q-type Ca2+ channel family. It is expressed preferentially in the central nervous system where along with CaV2.2 is responsible for pre-synaptic Ca2+ influx and neurotransmitter release.1,2
Mutations in CACNA1A (CaV2.1) have been shown to cause several neurological disorders among them are familial hemiplegic migraine, episodic ataxia type 2, and spinocerebellar ataxia type 6 (SCA6).1,3-5
The involvement of CaV2.1 in synaptic transmission was assessed by using ω-Agatoxin IVA (#STA-500), a specific blocker of the CaV2.1 channel.6 The blocking sensitivity is dependent on the α subunit isoform and on the splice variant.7,8
Application key:
Species reactivity key:
Anti-CACNA1A (CaV2.1) Antibody (#ACC-001) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize CaV2.1 from mouse, rat, and human samples.
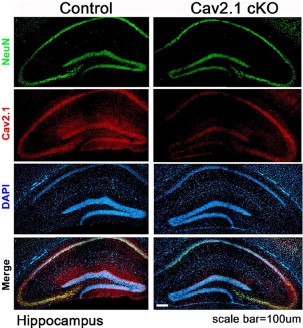
Expression of CaV2.1 in mouse hippocampus.Immunohistochemical staining of mouse hippocampal sections using Anti-CACNA1A (CaV2.1) Antibody (#ACC-001). CaV2.1 staining (red) is largely detected in the CA1 region and co-localizes with NeuN, a neuronal marker. Note the significant decrease in CaV2.1 detection in CaV2.1 conditional knockout mice (right panels).Adapted from Jung, D. et al. (2016) Front. Behav. Neurosci. 10, 214. with permission of Frontiers.
Applications
Citations
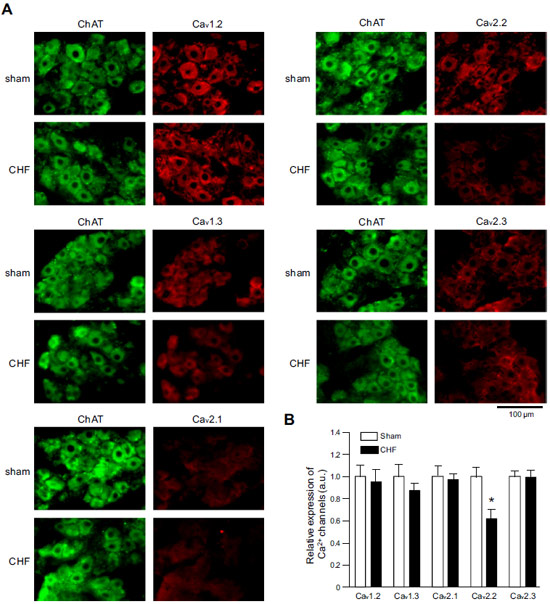 Expression of CaV α subunits in rat ICG.Immunohistochemical staining of rat intracardiac ganglia (ICG) using Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003), Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), Anti-CACNA1B (CaV2.2) Antibody (#ACC-002) and Anti-CaV2.3 (CACNA1E) Antibody (#ACC-006). All CaV subtypes are expressed in sham treated rats but N-type CaV channel levels are decreased in CHF rats.
Expression of CaV α subunits in rat ICG.Immunohistochemical staining of rat intracardiac ganglia (ICG) using Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003), Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), Anti-CACNA1B (CaV2.2) Antibody (#ACC-002) and Anti-CaV2.3 (CACNA1E) Antibody (#ACC-006). All CaV subtypes are expressed in sham treated rats but N-type CaV channel levels are decreased in CHF rats.
Adapted from Tu, H. et al. (2014) with permission of the American Physiological Society.
- Western blot analysis of mouse cerebellum lysate. Tested in CaV2.1 conditional knock out mice.
Dorgans, K. et al. (2017) Neurobiol. Dis. 106, 110. - Immunohistochemical staining of mouse brain sections. Tested in CaV2.1 conditional knock out mice.
Jung, D. et al. (2016) Front. Behav. Neurosci. 10, 214.
- Mouse cerebellum lysate. Also tested in CaV2.1 conditional knock out mice.
Dorgans, K. et al. (2017) Neurobiol. Dis. 106, 110. - Mouse brain lysate.
Valkova, C. et al. (2017) Sci. Rep. 7, 41248. - HEK 293 transfected cells (1:300).
Lopez Soto, E.J. et al. (2015) J. Gen. Physiol. 146, 205. - Rat primary submucosa cells.
Rehn, M. et al. (2013) Cell Tissue Res. 353, 355. - Human red blood cells.
Andrews, D.A. et al. (2002) Blood 100, 3392.
- Mouse brain sections. Also tested in CaV2.1 conditional knock out mice.
Jung, D. et al. (2016) Front. Behav. Neurosci. 10, 214. - Rat intracardiac ganglia and stellate ganglia neurons.
Tu, H. et al. (2014) Am. J. Physiol. 306, C132. - Human and mouse fetal lung sections (1:100).
Brennan, S.C. et al. (2013) PLoS ONE 8, e80294. - Rat hippocampus sections (electron microscopy).
Bucurenciu, I. et al. (2008) Neuron 57, 536. - Rat hippocampus sections.
Kulik, A. et al. (2004) Eur. J. Neurosci. 19, 2169.
- Rat DRG neuronal culture.
Leo, M. et al. (2017) Exp. Neurol. 288, 62. - Rat DRG transfected cells.
Dahimene, S. et al. (2016) Neurobiol. Dis. 93, 243. - Rat adipose-derived stromal cells and rat bone marrow stromal cells.
Forostyak, O. et al. (2016) Stem Cell Res. 16, 622. - Rat primary submucosa cells (1:75).
Rehn, M. et al. (2013) Cell Tissue Res. 353, 355. - Mouse sperm cells.
Serrano, C.J. et al. (1999) FEBS Lett. 462, 171.
- Fu, S.J. et al. (2017) J. Neurosci. 37, 2485.
- Liu, C.H. et al. (2016) Histochem. Cell Biol. 146, 599.
- Tang, W. et al. (2016) Front. Synaptic Neurosci. 8, 15.
- Tedeschi, A. et al. (2016) Neuron 92, 419.
- Alvarez, Y.D. et al. (2013) PLoS ONE 8, e54846.
- Tzour, A. et al. (2013) J. Neuroendocrinol. 25, 76.
- Christel, C.J. et al. (2012) J. Biol. Chem. 287, 39766.
- Lv, P. et al. (2012) J. Neurosci. 32, 163143.
- Ahmari, S.E. et al. (2000) Nat. Neurosci. 3, 445.
- Hansen, P.B. et al. (2000) Circ. Res. 87, 896.
- Restituito, S. et al. (2000) J. Neurosci. 20, 6394.
- Bae, I.H. et al. (1999) Korean J. Biol. Sci. 3, 53.
- Barbosa, J. et al. (1999) J. Neurochem. 73, 1881.
- Lopez, I. et al. (1999) Neuroscience 92, 773.
