Overview
|
| Bioz Stars Product Rating | |
| The world's only objective ratings for scientific research products | |
| Mentions | |
| Recency | |
| View product page > | |
Alomone Labs is pleased to offer a highly specific antibody directed against an extracellular epitope of rat CaVα2δ2. Anti-CACNA2D2 (CaVα2δ2) (extracellular) Antibody (#ACC-102) can be used in western blot and immunohistochemistry applications. It has been designed to recognize CaVα2δ2 from rat, mouse and human samples.
Application key:
Species reactivity key:
Applications
 Western blot analysis of rat lung (lanes 1 and 6), rat brain (lanes 2 and 7), mouse brain (lanes 3 and 8), rat heart (lanes 4 and 9) and mouse heart (lanes 5 and 10) lysates:1-5. Anti-CACNA2D2 (CaVα2δ2) (extracellular) Antibody (#ACC-102), (1:200).
Western blot analysis of rat lung (lanes 1 and 6), rat brain (lanes 2 and 7), mouse brain (lanes 3 and 8), rat heart (lanes 4 and 9) and mouse heart (lanes 5 and 10) lysates:1-5. Anti-CACNA2D2 (CaVα2δ2) (extracellular) Antibody (#ACC-102), (1:200).
6-10. Anti-CACNA2D2 (CaVα2δ2) (extracellular) Antibody, preincubated with CACNA2D2/Cavα2δ2 (extracellular) Blocking Peptide (#BLP-CC102).
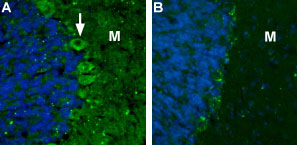 Expression of CaVα2δ2 channel in rat cerebellumImmunohistochemical staining rat cerebellum using Anti-CACNA2D2 (CaVα2δ2) (extracellular) Antibody (#ACC-102). A. CaVα2δ2 (green) appears in the soma of Purkinje cells (arrow) and in the molecular layer (M). B. Preincubation of the antibody with the blocking peptide blocks staining of Purkinje cells and molecular layer. DAPI is used as the counterstain (blue).
Expression of CaVα2δ2 channel in rat cerebellumImmunohistochemical staining rat cerebellum using Anti-CACNA2D2 (CaVα2δ2) (extracellular) Antibody (#ACC-102). A. CaVα2δ2 (green) appears in the soma of Purkinje cells (arrow) and in the molecular layer (M). B. Preincubation of the antibody with the blocking peptide blocks staining of Purkinje cells and molecular layer. DAPI is used as the counterstain (blue).
Citations (111)
- Rat TG and dorsal Vc/C2 lysates (1:200).
Li, K.W. et al. (2014) J. Biol. Chem. 289, 7025. - Xenopus oocytes expressing α2δ2 subunit.
Edvardson, S. et al. (2013) J. Med. Genet. 50, 118.
Specifications
- (C)DLEAWAEKFKVLASNR, corresponding to amino acid residues 850-865 of rat CaVα2δ2 (Accession Q8CFG6). Extracellular, N-terminus.

Scientific Background
Voltage-gated Ca2+ channels (CaV), enable the passage of Ca2+ ions in a voltage-dependent manner. These heteromeric entities are formed in part by the pore-forming α1 subunit which determines the biophysical and pharmacological properties of the channel1.
CaV1 and CaV2 channels are high-voltage activated (HVA) CaV channels. The a1 subunit of these channels normally interacts and associates with α2δ subunit, a membrane anchored protein and CaVβ, a cytosolic protein1.
Four α2δ subunits have been cloned to date: α2δ1-4. This subunit originates from a single gene. The corresponding protein is modified by post-translational cleavage yielding a α2 subunit which is disulfide bonded to the δ subunit2. All α2δ subunits are GPI- (glycosylphosphatidylinositol) anchored proteins3. The role of this subunit is important for the membrane trafficking of the α1 subunit, and also has a role in influencing the biophysical properties of the channel1.
α2δ can be expressed as various splice variants and expressed in a tissue specific manner. α2δ2 can be detected in the brain, heart, lung, spleen and liver4.
Gabapentin and pregabalin are two commonly used anti-epileptic drugs. They act on CaV channels via the α2δ1 and α2δ2 subunits by disturbing their membrane trafficking, thereby decreasing Ca2+ currents.
- Bauer, C.S. et al. (2010) Curr. Opin. Neurobiol. 20, 563.
- Jay, S.D. et al. (1991) J. Biol. Chem. 266, 3287.
- Davies, A. et al. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 1654.
- Klugbauer, N. et al. (2003) J. Bioenerg. Biomembr. 35, 639.

