Overview
- Peptide MDEEEDGAGAEESGQPRSFTQL(C), corresponding to amino acid residues 1-22 of rat CACNA1G (Accession O54898). Intracellular, N-terminus.
- Rat brain membranes (1:200).
 Western blot analysis of rat brain membranes:1. Anti-CACNA1G (CaV3.1) Antibody (#ACC-021), (1:200).
Western blot analysis of rat brain membranes:1. Anti-CACNA1G (CaV3.1) Antibody (#ACC-021), (1:200).
2. Anti-CACNA1G (CaV3.1) Antibody, preincubated with CACNA1G/Cav3.1 Blocking Peptide (#BLP-CC021).
- Human myometrium cells (1:200) (Blanks, A.M. et al. (2007) J. Physiol. 581, 915.).
Voltage-dependent Ca2+ channels provide a pathway for rapid influx of Ca2+ into cells, which plays a crucial role in both electrical and metabolic signaling.1
T-type currents are transduced via channel proteins encoded by three genes that compose a subfamily within the CaV channel family.2-3
The activity of T-type channels contributes to several known physiological and pathophysiological phenomena including burst firing in neurons, pacemaking activity in the heart and secretion from endocrine tissues.2 There are three cloned T-type channel isoforms.
CACNA1G (CaV3.1) and CACNA1H (CaV3.2) are widely distributed whereas the expression of CACNA1I (CaV3.3) is restricted to the central nervous system.2
CACNA1G and CACNA1H are also expressed in the kidney, but little is known about their physiological role there.
Application key:
Species reactivity key:
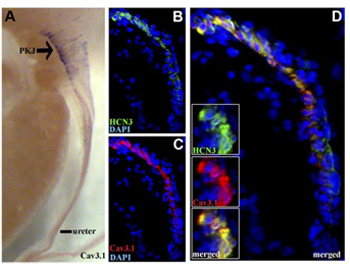
Expression of CaV3.1 in mouse pelvis kidney junction.
Immunohistochemical staining of mouse kidney sections using Anti-CACNA1G (CaV3.1) Antibody (#ACC-021). A. CaV3.1 (purple) is detected in the pelvis kidney junction (PKJ). B. HCN3 staining (green), a marker of pacemaker cells, is detected in the PKJ. C. Same section stained for CaV3.1 (red). D. Merge of B and C panels shows high degree of co-localization between CaV3.1 and HCN3.
Adapted from Hurtado, R. et al. (2014) with permission of Federation of American Societies for Experimental Biology.
