Overview
- Peptide CHVEGPQERARVAHS, corresponding to amino acid residues 581-595 of rat CaV3.2 (Accession number Q9EQ60). Intracellular loop between domains D1 and D2.

 Western blot analysis of rat DRG lysates:1. Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025), (1:200).
Western blot analysis of rat DRG lysates:1. Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025), (1:200).
2. Anti-CaV3.2 (CACNA1H) Antibody, preincubated with Cav3.2/CACNA1H Blocking Peptide (#BLP-CC025).- Human CaV3.2 transfected in HEK-293 cells (1:100) (Markandeya, Y.S. et al. (2011) J. Biol. Chem. 286, 2433.).
- Rat brain lysates (5 μg) (Weiss, N. et al. (2012) J. Biol. Chem. 287, 2810.).
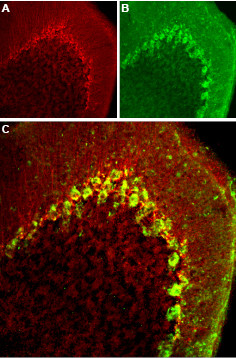 Expression of CaV3.2 in mouse cerebellumImmunohistochemical staining of mouse cerebellum frozen sections with Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025), (1:100). A. CaV3.2 appears adjacent to Purkinje cells and in fibers in the molecular layer (red). B. Staining of Purkinje cells with mouse anti-parvalbumin (PV, green). C. Merged image of panels A and B demonstrates presence of CaV3.2 adjacent to Purkinje cells.
Expression of CaV3.2 in mouse cerebellumImmunohistochemical staining of mouse cerebellum frozen sections with Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025), (1:100). A. CaV3.2 appears adjacent to Purkinje cells and in fibers in the molecular layer (red). B. Staining of Purkinje cells with mouse anti-parvalbumin (PV, green). C. Merged image of panels A and B demonstrates presence of CaV3.2 adjacent to Purkinje cells. Expression of CaV3.2 in rat DRGImmunohistochemical staining of rat dorsal root ganglion (DRG) frozen sections with Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025), (1:50). Staining is specific for DRG. Note that neither glial cells nor axonal fibers are stained. Hoechst 33342 is used as the counterstain.
Expression of CaV3.2 in rat DRGImmunohistochemical staining of rat dorsal root ganglion (DRG) frozen sections with Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025), (1:50). Staining is specific for DRG. Note that neither glial cells nor axonal fibers are stained. Hoechst 33342 is used as the counterstain.
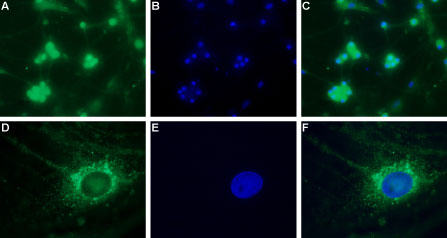 Expression of CaV3.2 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized rat dorsal root ganglion (DRG) primary culture.
Expression of CaV3.2 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized rat dorsal root ganglion (DRG) primary culture.
A, D. Immunocytochemical staining using Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025), (1:200), followed by goat anti-rabbit-AlexaFluor-488 secondary antibody.
B, E. Nuclear fluorescence staining of cells using the membrane-permeable DNA dye Hoechst 33342.
C. Merged image of panels A and B.
F. Merged image of panels D and E.
Magnification:
A-C: x20
D-F: x100
- Park, J.Y. et al. (2004) J. Biol. Chem. 279, 21707.
- Chemin, J. et al. (2001) Eur. J. Neurosci. 14, 1678.
- Monteil, A. et al. (2000) J. Biol. Chem. 275, 16530.
- Gackière, F. et al. (2008) J. Biol. Chem. 283, 10162.
- Khosravani, H. et al. (2004) J. Biol. Chem. 279, 9681.
- Chen, C.C. et al. (2003) Science 302,1416.
- Bourinet, E. et al. (2005) EMBO J. 24, 315.
T-type Ca2+ channels play an important role in many cellular processes such as hormone secretion, neurotransmitter release and cell differentiation. T-type channels are also known to participate in the pacemaker activities of the heart and neurons including thalamic neurons.1
Three genes encoding T-type Ca2+ channels have been cloned and designated as CaV3.2 (CACNA1H), CaV3.1 (CACNA1G) and CaV3.3 (CACNA1I).1-3
The CaV3.2 channel is widely expressed in various tissues such as the brain, heart, liver and testis.
Involvement of CaV3.2 Ca2+ channels in several pathologies has been described. Overexpression of the CaV3.2 channel was described in prostate cancer cells that are associated with more aggressiveness, invasiveness and poor prognosis.4 Several point mutations discovered in the CaV3.2 channel that affect gating of the channel were found to be associated with Childhood Absence Epilepsy.5 One year old mice, deficient in CaV3.2 channel, exhibited severe cardiac pathology, fibrosis, necrosis, lymphocyte infiltration and abnormal coronary function compared to wild-type mice.6
Recently, it has been demonstrated that T-type channels are expressed in DRG neurons and that small and medium-diameter primary afferent neurons in the dorsal horn expresses almost exclusively the CaV3.2 Ca2+channels. This might indicate a possible role for CaV3.2 in nociception.7
Application key:
Species reactivity key:
Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunoprecipitation, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize CaV3.2 from rat, human, and mouse samples.
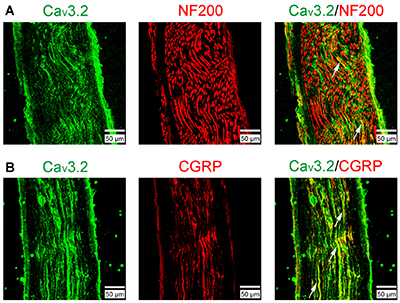
Expression of CaV3.2 in Rat Sural Nerve.Immunohistochemical staining of rat sural nerve sections using Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025). A. CaV3.2 staining (green) partially colocalizes with neurofilament 200. B. CaV3.2 staining (green) significantly colocalizes with calcitonin gene-related peptide (CGRP, red), a marker for nociceptive peptidergic fibers.Adapted from Chen, W. et al. (2018) Front. Mol. Neurosci. 11, 24. with permission of Frontiers.
Applications
Citations
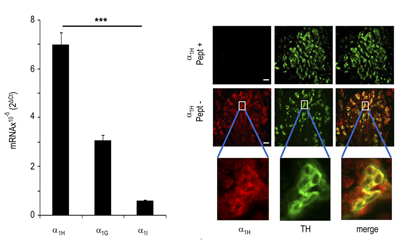 Expression of CaV3.2 in rat carotid body.Left: mRNA expression of different CaV channels in the carotid body. Right: Immunohistochemical staining of rat carotid body using Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025). CaV3.2 staining (red) is detected in glomus cells. Use of the control peptide antigen eliminated the signal obtained using the antibody.
Expression of CaV3.2 in rat carotid body.Left: mRNA expression of different CaV channels in the carotid body. Right: Immunohistochemical staining of rat carotid body using Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025). CaV3.2 staining (red) is detected in glomus cells. Use of the control peptide antigen eliminated the signal obtained using the antibody.
Adapted from Makarenko, V.V. et al. (2015) with permission of the American physiological society.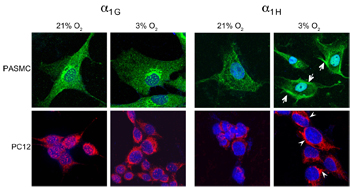 Upregulation of CaV3.2 under hypoxia.Immunocytochemical staining of rat pulmonary artery smooth muscle cells (PASMCs) and rat pheochromocytoma cells (PC12) using Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025) and Anti-CACNA1G (CaV3.1) Antibody (#ACC-021). Staining shows upregulation of CaV3.2 and not CaV3.1 in response to oxygen stress.
Upregulation of CaV3.2 under hypoxia.Immunocytochemical staining of rat pulmonary artery smooth muscle cells (PASMCs) and rat pheochromocytoma cells (PC12) using Anti-CaV3.2 (CACNA1H) Antibody (#ACC-025) and Anti-CACNA1G (CaV3.1) Antibody (#ACC-021). Staining shows upregulation of CaV3.2 and not CaV3.1 in response to oxygen stress.
Adapted from Sellak, H. et al. (2014) with permission of the American Physiological Society.
- Western blot analysis of mouse brain lysate. Tested in CACNA1H-/- mice.
Proft, J. et al. (2017) Sci. Rep. 7, 11513.
- Mouse brain lysate. Also tested in CACNA1H-/- mice.
Proft, J. et al. (2017) Sci. Rep. 7, 11513. - HEK 293 cell lysates (1:500).
Makarenko, V.V. et al. (2016) J. Neurophysiol. 115, 345. - Human artery lysates (1:200).
Harraz, O.F. et al. (2015) J. Gen. Physiol. 145, 405. - Rat DRG lysates (1:200).
Makarenko, V.V. et al. (2015) Am. J. Physiol. 308, C-146. - Rat hippocampus lysate (1:1500).
Sanchez-Aguilera, A. et al. (2014) J. Physiol. 592, 2845. - Mouse lung and brain lysate (1:200).
Wan, J. et al. (2013) Am. J. Physiol. 305, L154. - Human CaV3.2 transfected in HEK-293 cells (1:100).
Markandeya, Y.S. et al. (2011) J. Biol. Chem. 286, 2433.
- Rat brain lysates (5 μg).
Weiss, N. et al. (2012) J. Biol. Chem. 287, 2810.
- Rat sural nerve sections.
Chen, W. et al. (2018) Front. Mol. Neurosci. 11, 24. - Rat DRG sections (1:100).
Liu, Z. et al. (2016) Mol. Pain 12, 1. - Rat retinal whole mounts (1:200).
Fernandez, J.A. et al. (2015) Invest. Ophtalmol. Vis. Sci. 56, 5125. - Rat carotid sections (1:200).
Makarenko, V.V. et al. (2015) Am. J. Physiol. 308, C-146.
- Rat DRGs (1:200).
Huang, D. et al. (2016) Biochem. Biophys. Res. Commun. 473, 693. - Human NCI-H295R transfected cells (1:500).
Reimer, E.N. et al. (2016) Endocrinology 157, 3016. - Rat pulmonary artery smooth muscle cells (PASMCs) and rat pheochromocytoma cells (PC12), (1:100).
Sellak, H. et al. (2014) Am. J. Physiol. 307, C648.
- Abd El-Rahman, R.R. et al. (2013) Am. J. Physiol. 304, H58.
- Favereaux, A. et al. (2011) EMBO J. 30, 3830.
- Anderson, D. et al. (2010) Nat. Neurosci. 13, 333.
- Levitsky, K.L. and López-Barneo, J. (2009) J. Physiol. 587.9, 1917.
- Taylor, J.T. et al. (2008) Cancer Lett. 267, 116.
