Overview
- Peptide (C)SESTVNVLKMIRS, corresponding to amino acid residues 87-99 of rat CACNG5 (Accession Q8VHW8). 1st extracellular loop.
- Rat and mouse brain lysates (1:1000-1:5000).
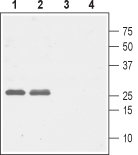 Western blot analysis of rat (lanes 1 and 3) and mouse (lanes 2 and 4) brain lysates:1,2. Anti-CACNG5 (extracellular) Antibody (#ACC-115), (1:1000).
Western blot analysis of rat (lanes 1 and 3) and mouse (lanes 2 and 4) brain lysates:1,2. Anti-CACNG5 (extracellular) Antibody (#ACC-115), (1:1000).
3,4. Anti-CACNG5 (extracellular) Antibody, preincubated with CACNG5 (extracellular) Blocking Peptide (#BLP-CC115).
- Living intact rat PC12 cells (1:25).
Voltage-gated Ca2+ (CaV) channels are ubiquitously expressed and function as Ca2+ conducting pores in the plasma membrane1. On the basis of their voltage activation properties, the voltage-gated Ca2+ channels can be further divided into two broad groups: the low (T-type) and high (L, N, P, Q and R-type) threshold-activated channels.2. CaV channels are heteromultimers composed of four independently encoded proteins, the pore-forming α1 subunit, which triggers Ca2+ flow across the membrane, and the auxiliary subunits α2δ, γ, and β3.
CaVγ subunits inhibit CaV channel activity and modulate its activation and inactivation kinetics. CaVγ subunits have little effect on CaV channel trafficking4. The γ subunit is an integral membrane protein. The γ subunit family consists of at least 8 members, which share a number of common structural features. Each member is predicted to possess four transmembrane domains, with intracellular N- and C-termini. The first extracellular loop contains a highly conserved N-glycosylation site and a pair of conserved cysteine residues5.
The CaVγ5 subunit is highly expressed in the liver, kidney, heart, lung, skeletal muscles, and, with a lower abundance, in testes5. The close association of epilepsy and ataxia with mutations in other neuronal voltage-dependent Ca2+ channels suggests these are potential candidate phenotypes for defects in the CaVγ5 (CACNG5) gene6.
