Overview
- Peptide (C)KEETEEEVQDTRL, corresponding to amino acid residues 1468-1480 of human CFTR (Accession P13569). Cytoplasmic, C-terminal part.
- Rat lung membranes (1:200).
Mouse caveolar fraction lysate (1:500) (Tabeling, C. et al. (2015) Proc. Natl. Acad. Sci. U.S.A. 112, E1614.) 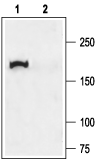 Western blot analysis of rat lung membranes:1. Anti-CFTR Antibody (#ACL-006), (1:200).
Western blot analysis of rat lung membranes:1. Anti-CFTR Antibody (#ACL-006), (1:200).
2. Anti-CFTR Antibody, preincubated with CFTR Blocking Peptide (#BLP-CL006).
- Mouse embryonic stem cells (Liu, Z. et al. (2017) Cell Death Differ. 24, 98.).
- Rat lung sections.
- Mouse embryonic stem cells (Liu, Z. et al. (2017) Cell Death Differ. 24, 98.).
The cystic fibrosis transmembrane conductance regulator (CFTR) is the most dominant Cl- channel in several epithelial tissues, especially in lung and colon. Remarkably, CFTR is a member of the ATP-binding cassette (ABC) transporter superfamily that uses ATP hydrolyzation as the driving force for the translocation of a wide variety of substrates including sugars, amino acids, proteins and hydrophobic compounds, across cellular membranes. The CFTR is unique among ABC transporters in that it is a cAMP-regulated Cl- channel. It shares the superfamily topology of 12 transmembrane domains with two nucleotide-binding domains (NBDs) and a regulatory (R) domain in the large third intracytoplasmic loop that is phosphorylated in multiple sites by PKA. Mutations in the CFTR gene cause channel dysfunction in several ways, ranging from complete loss of surface expression to diminished Cl- secretion. Defects in the CFTR gene cause cystic fibrosis (CF), the most common genetic disease among Caucasians, as well as a form of male sterility.
Regulation of the CFTR channel is accomplished through the activation of surface receptors that couple to adenyl cyclase, raise cAMP cellular levels and thus activate PKA. This has been demonstrated for the adenosine and ß2 adrenergic receptor and the vasopressin hormone among others.
Besides enhanced Cl- conductance, activation of CFTR also leads to the regulation of other ion channels. The best-studied case is its interaction with the epithelial Na+ channels (ENaC), although it can probably regulate other ion channels as well (Kir1.1 for example). The mechanism by which CFTR regulates other ion channels is not clear, but it may involve protein-protein interactions via molecules that interact with its C-terminal PDZ binding motif, such as the NHERF adaptor protein.
Application key:
Species reactivity key:
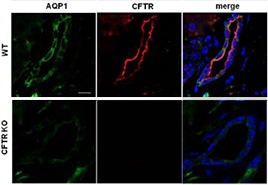
Knockout validation of Anti-CFTR Antibody in mouse pancreas.Immunohistochemical staining of mouse pancreas sections using Anti-CFTR Antibody (#ACL-006). CFTR immunoreactivity (red) is exclusively localized to apical membranes in the ducts. AQP1 staining (green) is observed throughout the plasma membrane. CFTR is not detected in CFTR-/- mice (bottom pannels).Adapted from Venglovecz, V. et al. (2018) Front. Physiol. 9, 854. with permission of Frontiers.
