Overview
- Peptide (C)KEETEEEVQDTRL, corresponding to amino acid residues 1468-1480 of human CFTR (Accession P13569). Cytoplasmic, C-terminal part.

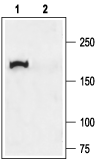 Western blot analysis of rat lung membranes:1. Anti-CFTR Antibody (#ACL-006), (1:200).
Western blot analysis of rat lung membranes:1. Anti-CFTR Antibody (#ACL-006), (1:200).
2. Anti-CFTR Antibody, preincubated with CFTR Blocking Peptide (#BLP-CL006).- Mouse caveolar fraction lysate (1:500) (Tabeling, C. et al. (2015) Proc. Natl. Acad. Sci. U.S.A. 112, E1614.)
- Mouse embryonic stem cells (Liu, Z. et al. (2017) Cell Death Differ. 24, 98.).
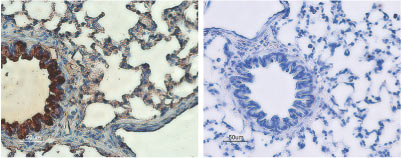 Expression of CFTR in rat lungsImmunohistochemical staining of rat lungs sections using Anti-CFTR Antibody (#ACL-006) (left panel). Strong staining of bronchial epithelial cells (red) and lighter staining of alveolar cells (red-brown) is apparent. There is also positive staining of macrophages while smooth muscle and endothelium are negative. Counterstain of cell nuclei appears blue. A negative control is shown in the right panel.
Expression of CFTR in rat lungsImmunohistochemical staining of rat lungs sections using Anti-CFTR Antibody (#ACL-006) (left panel). Strong staining of bronchial epithelial cells (red) and lighter staining of alveolar cells (red-brown) is apparent. There is also positive staining of macrophages while smooth muscle and endothelium are negative. Counterstain of cell nuclei appears blue. A negative control is shown in the right panel.
- Mouse embryonic stem cells (Liu, Z. et al. (2017) Cell Death Differ. 24, 98.).
- Jentsch, T.J. et al. (2002) Physiol. Rev. 82, 503.
- Pilewski J.M. et al. (1999) Physiol Rev 79, S215–S255.
- Greger, R. et al. (2001) Pflugers Arch. 443 (Suppl. 1), S3-S7.
The cystic fibrosis transmembrane conductance regulator (CFTR) is the most dominant Cl- channel in several epithelial tissues, especially in lung and colon. Remarkably, CFTR is a member of the ATP-binding cassette (ABC) transporter superfamily that uses ATP hydrolyzation as the driving force for the translocation of a wide variety of substrates including sugars, amino acids, proteins and hydrophobic compounds, across cellular membranes. The CFTR is unique among ABC transporters in that it is a cAMP-regulated Cl- channel. It shares the superfamily topology of 12 transmembrane domains with two nucleotide-binding domains (NBDs) and a regulatory (R) domain in the large third intracytoplasmic loop that is phosphorylated in multiple sites by PKA. Mutations in the CFTR gene cause channel dysfunction in several ways, ranging from complete loss of surface expression to diminished Cl- secretion. Defects in the CFTR gene cause cystic fibrosis (CF), the most common genetic disease among Caucasians, as well as a form of male sterility.
Regulation of the CFTR channel is accomplished through the activation of surface receptors that couple to adenyl cyclase, raise cAMP cellular levels and thus activate PKA. This has been demonstrated for the adenosine and ß2 adrenergic receptor and the vasopressin hormone among others.
Besides enhanced Cl- conductance, activation of CFTR also leads to the regulation of other ion channels. The best-studied case is its interaction with the epithelial Na+ channels (ENaC), although it can probably regulate other ion channels as well (Kir1.1 for example). The mechanism by which CFTR regulates other ion channels is not clear, but it may involve protein-protein interactions via molecules that interact with its C-terminal PDZ binding motif, such as the NHERF adaptor protein.
Application key:
Species reactivity key:
Anti-CFTR Antibody (#ACL-006) is a highly specific antibody directed against an epitope of the human protein. The antibody can be used in western blot, immunoprecipitation, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize CFTR from mouse, rat, and human samples.
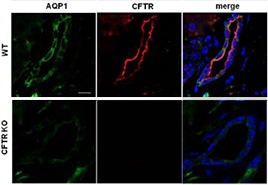
Knockout validation of Anti-CFTR Antibody in mouse pancreas.Immunohistochemical staining of mouse pancreas sections using Anti-CFTR Antibody (#ACL-006). CFTR immunoreactivity (red) is exclusively localized to apical membranes in the ducts. AQP1 staining (green) is observed throughout the plasma membrane. CFTR is not detected in CFTR-/- mice (bottom pannels).Adapted from Venglovecz, V. et al. (2018) Front. Physiol. 9, 854. with permission of Frontiers.
Applications
Citations
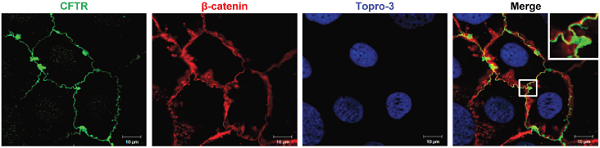 Expression of CFTR in human Caco-2 cellsImmunocytochemical staining of human epithelial colorectal adenocarcinoma cells using Anti-CFTR Antibody (#ACL-006). CFTR (green) co-localizes with β-Catenin (red). To-Pro3 is used to stain nuclei.
Expression of CFTR in human Caco-2 cellsImmunocytochemical staining of human epithelial colorectal adenocarcinoma cells using Anti-CFTR Antibody (#ACL-006). CFTR (green) co-localizes with β-Catenin (red). To-Pro3 is used to stain nuclei.
Adapted from Liu, K. et al. (2016) Oncotarget 7, 64030. with permission of Impact Journals.
- Immunohistochemical staining of mouse pancreas sections. Tested in CFTR-/- mice.
Venglovecz, V. et al. (2018) Front. Physiol. 9, 854.
- Mouse colon epithelia lysate.
Rottgen, T.S. et al. (2018) Am. J. Physiol. 315, C10. - Mouse embryonic stem cells.
Liu, Z. et al. (2017) Cell Death Differ. 24, 98. - Rat ovary lysates (1:200).
Chen, H. et al. (2015) Reproduction 149, 393. - Mouse caveolar fraction lysate (1:500).
Tabeling, C. et al. (2015) Proc. Natl. Acad. Sci. U.S.A. 112, E1614. - Mouse uterus lysate (1:1200).
Zhou, M. et al. (2014) PLoS ONE 9, e99521.
- Mouse embryonic stem cells.
Liu, Z. et al. (2017) Cell Death Differ. 24, 98.
- Mouse pancreas sections. Also tested in CFTR-/- mice.
Venglovecz, V. et al. (2018) Front. Physiol. 9, 854. - Mouse tendon sections.
Liu, Y. et al. (2017) FASEB J. 31, 3800. - Rat ovary sections (1:50).
Chen, H. et al. (2015) Reproduction 149, 393. - Mouse uterus sections (1:1000).
Zhou, M. et al. (2014) PLoS ONE 9, e99521. - Mouse lung sections (1:500).
Li, S. and Xiang, M. (2011) Dev. Dyn. 240, 1512.
- Chen, J. et al. (2012) J. Cell. Physiol. 227, 2759.
- Merigo, F. et al. (2011) Dev. Neurobiol. 71, 854.
- Sorio, C. et al. (2011) PLoS One 6, e22212.
- Chen, M.H. et al. (2010) Hum. Reprod. 25, 1744.
- Merigo, F. et al. (2009) Cell Tissue Res. 336, 411.
- Chen, M. et al. (2008) Acta Biochim. Biophys. Sin. 40, 864.
- Ishibashi, K. et al. (2008) Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1729.
- Merigo, F. et al. (2008) Chem. Senses 33, 231.
- Pietrement, C. et al. (2008) J. Biol. Chem. 283, 2986.
- Li, J. et al. (2007) J. Biol. Chem. 282, 27086.
- Xu, W.M. et al. (2007) Proc. Natl. Acad. Sci. USA 104, 9816.
- Ishibashi, K. et al. (2006) J. Dent. Res. 85, 1101.
- Li, J. et al. (2005) J. Biol. Chem. 45, 37634.
