Overview
- Peptide RSRHGLPREGTPSDSDDKC, corresponding to amino acid residues 888-906 of rat CLC-2. (Accession P35525). Intracellular, C-terminus.
 Western blot analysis of rat brain membranes:1. Anti-CLC-2 (CLCN2) Antibody (#ACL-002), (1:200).
Western blot analysis of rat brain membranes:1. Anti-CLC-2 (CLCN2) Antibody (#ACL-002), (1:200).
2. Anti-CLC-2 (CLCN2) Antibody, preincubated with CLC-2/CLCN2 Blocking Peptide (#BLP-CL002).
- Rat brain membranes.
- Human fibroblasts (1:100) (Lakoma, J. et al. (2016) J. Cell. Physiol. 231, 192.).
- Jentsch, T.J. et al. (2002) Physiol. Rev. 82, 503.
- Babini, E. and Pusch, M. (2004) Physiology 19, 293.
- Haug, K. et al. (2003) Nat. Genet. 33, 527.
- Bosl, M.R. et al. (2001) EMBO J 20, 1289.
CLC-2 is a member of the voltage-dependent Cl- channel (CLC) family that includes nine known members in mammals. CLC channels can be classified as plasma membrane channels and intracellular organelle channels. The first group includes the CLC-1, CLC-2 CLC-Ka and CLCKb channels. The second group comprises the CLC-3, CLC-4, CLC-5, CLC-6 and CLC-7.
CLC channels that function in the plasma membrane are involved in the stabilization of membrane potential and in transepithelial transport. The presumed function of the intracellular CLC channels is support of the acidification of the intraorganellar compartment. In this regard, recent reports indicate that ClC-4 and ClC-5 (and by inference ClC-3) can function as Cl-/H+ antiporters.1, 2
The functional unit of the CLC channels is a dimer with each subunit forming a proper pore. Although the crystal structure of bacterial CLC channels was resolved, the topology of the CLC channels is complex and has not been fully elucidated. It is generally accepted that both the N- and C- terminus domains are intracellular while the number and configuration of the transmembrane domains vary greatly between different models. 1,2
CLC-2 is widely distributed with prominent expression in brain, kidney, lung and the gastrointestinal system. Mutations in the CLC-2 channel gene detected in humans are associated with idiopathic generalized epilepsies while disruption of the ClC-2 gene in mice is associated with testicular and retinal degeneration. 3,4
Application key:
Species reactivity key:
Anti-CLC-2 (CLCN2) Antibody (#ACL-002) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunohistochemistry and immunocytochemistry applications. It has been designed to recognize CLC-2 channel from rat, mouse, and human samples.
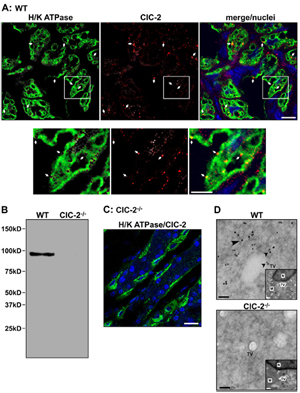
Knockout validation of Anti-CLC-2 (CLCN2) Antibody in parietal cells of mouse gastric mucosa.A. Immunohistochemical staining of mouse gastric mucosa using Anti-CLC-2 (CLCN2) Antibody (#ACL-002) (red). Same sections are stained for H+/K+ ATPase (green). Merge panel shows co-localization of the two proteins. B. Western blot analysis of ClC-2 in WT and ClC-2-/- mouse gastric mucosa using Anti-CLC-2 (CLCN2) Antibody shows that antibody is indeed specific for ClC-2. C. Immunohistochemical staining of mouse gastric mucosa shows lack of ClC-2 staining in ClC-2-/- mouse. D. Immunogold electronmicroscopy of ClC-2 in WT and ClC-2-/- mouse gastric parietal cells.Adapted from Nighot, M.P. et al. (2015) PLoS ONE 10, e0138174. with kind permission of Dr. Blikslager, A.T. North Carolina State University, College of Veterinary Medicine, Raleigh, North Carolina, United States of America.
Applications
Citations
- Western blot analysis of mouse gastric mucosa lysate. Immunohistochemical staining of mouse gastric tissue sections. Also tested in ClC-2-/- mouse gastric parietal cells.
Nighot, M.P. et al. (2015) PLoS ONE 10, e0138174.
- Human fibroblasts (1:200).
Lakoma, J. et al. (2016) J. Cell. Physiol. 231, 192. - Western blot analysis of mouse gastric mucosa lysate. Also tested in ClC-2-/- mouse gastric parietal cells.
Nighot, M.P. et al. (2015) PLoS ONE 10, e0138174.
- Mouse gastric tissue sections. Also tested in ClC-2-/- mouse gastric parietal cells.
Nighot, M.P. et al. (2015) PLoS ONE 10, e0138174. - Mouse nasal cavity sections.
Schiffhauer, E.S. et al. (2013) Am. J. Physiol. 304, L324.
- Human fibroblasts (1:100).
Lakoma, J. et al. (2016) J. Cell. Physiol. 231, 192.
- Catalán, M.A. et al. (2012) Gastroenterology 142, 346.
- Ao, M. et al. (2011) Dig. Dis. Sci. 56, 339.
- Ge, Y.X. et al. (2011) Neuroscience 186, 120.
- Inagaki, A. et al. (2010) J. Membr. Biol. 235, 27.
- Huang, Z.M. et al. (2009) J. Mol. Cell. Cardiol. 47, 121.
- Romanenko, V.G. et al. (2008) Am. J. Physiol. 295, G1058.
- Benfenati, V. et al. (2007) J. Neurochem. 100, 87.
- Hinzpeter, A. et al. (2007) J. Biol. Chem. 282, 2423.
- Rose, U. et al. (2007) J. Exp. Biol. 210, 2489.
- Britton, F.C. et al. (2005) J. Biol. Chem. 280, 25871.
- Ernest, N.J. et al. (2005) Am. J. Physiol. 288, C1451.
- Peña-Munzenmayer, G. et al. (2005) J. Cell Sci. 118, 4243.
- Fadool, D.A. et al. (2004) Neuron 41, 389.
- Koni, P.A. et al. (2003) J. Biol. Chem. 278, 39443.
- Olsen, M.L. et al. (2003) J. Neurosci. 23, 5572.
- DeFazio, R.A. et al. (2002) Mol. Endocrinol. 16, 2872.
- Sherry, A.M. et al. (2001) Am. J. Physiol. 280, C1599.
- Britton, F.C. et al. (2000) Am. J. Physiol. 279, H2225.
- Wills, N.K. et al. (2000) Invest. Ophtalmol. Vis. Sci. 41, 4247.
