Overview
- GST fusion protein with the sequence SLVVIVFELTGGLEYIVPLMAAVMTSKWVGDAFGREGIYEAHIRLNGYPFLDAKEEFTHTTLAADVMRPR, corresponding to amino acid residues 592-661 of rat CLC-3 (Accession P51792). Intracellular, near the C-terminus.
Rat CLC-4 - 46/70 amino acid residues identical; rat CLC-5 - 49/70 amino acid residues identical.
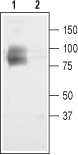 Western blot analysis of rat brain membranes:1. Anti-CLC-3 (CLCN3) Antibody (#ACL-001), (1:200).
Western blot analysis of rat brain membranes:1. Anti-CLC-3 (CLCN3) Antibody (#ACL-001), (1:200).
2. Anti-CLC-3 (CLCN3) Antibody, preincubated with CLC-3/CLCN3 Blocking peptide (#BLP-CL001). Western blot analysis of membranes from Xenopus oocytes, expressing CLC-3, CLC-4, and CLC-5, using Anti-CLC-3 (CLCN3) Antibody (#ACL-001) (kindly provided by Prof. Jordi Ehrenfeld, University of Nice).
Western blot analysis of membranes from Xenopus oocytes, expressing CLC-3, CLC-4, and CLC-5, using Anti-CLC-3 (CLCN3) Antibody (#ACL-001) (kindly provided by Prof. Jordi Ehrenfeld, University of Nice).- Human glioma lysates (1:500), (Cuddapah, V.A. et al. (2013) J. Neurosci. 33, 1427.).
- tsA cells expressing CLC-3 (Farmer, L.M. et al. (2013) J. Physiol. 591, 1001.).
- Rat brain sections (1:100) (Farmer, L.M. et al. (2013) J. Physiol. 591, 1001.).
- tsA cells expressing CLC-3 (Farmer, L.M. et al. (2013) J. Physiol. 591, 1001.).
- Jentsch, T.J. et al. (2002) Physiol. Rev. 82, 503.
- Babini, E. and Pusch, M. (2004) Physiology 19, 293.
- Stobrawa, S.M. et al. (2001) Neuron 29, 185.
- Moreland, J.G. et al. (2006) J. Biol. Chem. 281, 12277.
CLC-3 is a member of the voltage-dependent Cl- channel (CLC) family that includes nine known members in mammals. CLC channels can be classified as plasma membrane channels and intracellular organelle channels. The first group includes the CLC-1, CLC-2 CLC-Ka and CLCKb channels. The second group comprises the CLC-3, CLC-4, CLC-5, CLC-6 and CLC-7.
CLC channels that function in the plasma membrane are involved in the stabilization of membrane potential and in transepithelial transport. The presumed function of the intracellular CLC channels is support of the acidification of the intraorganellar compartment. In this regard, recent reports indicate that ClC-4 and ClC-5 (and by inference ClC-3) can function as Cl-/H+ antiporters.1, 2
The functional unit of the CLC channels is a dimer with each subunit forming a proper pore. Although the crystal structure of bacterial CLC channels was resolved, the topology of the CLC channels is complex and has not been fully elucidated. It is generally accepted that both the N- and C- terminus domains are intracellular while the number and configuration of the transmembrane domains vary greatly between different models. 1,2
CLC-3 is widely distributed with prominent expression in tissues of neuroectoderm origin. In the brain, it is highly expressed in the hippocampus, olfactory bulb and olfactory cortex. The channel is also prominently expressed in aortic and coronary vascular smooth muscle cells, aortic endothelial cells and tracheal and alveolar epithelial cells.
The physiological function of CLC-3 is not entirely clear, but it has been suggested that CLC-3 generates a shunt current of chloride for v-H+-ATPases, thereby aiding the acidification of endosomes and synaptic vesicles as well as lysosomes. Disruption of the ClC-3 gene in mice causes severe neuronal loss, leading to a complete loss of the hippocampus in adult mice. In addition, CLC-3 has been shown to have a critical role in the respiratory burst and phagocytosis of polymorphonuclear cells, a key cell type of innate host defense. 3,4
Application key:
Species reactivity key:
Anti-CLC-3 (CLCN3) Antibody (#ACL-001) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot and immunohistochemistry applications. It has been designed to recognize CLC-3 from rat, mouse, and human samples.
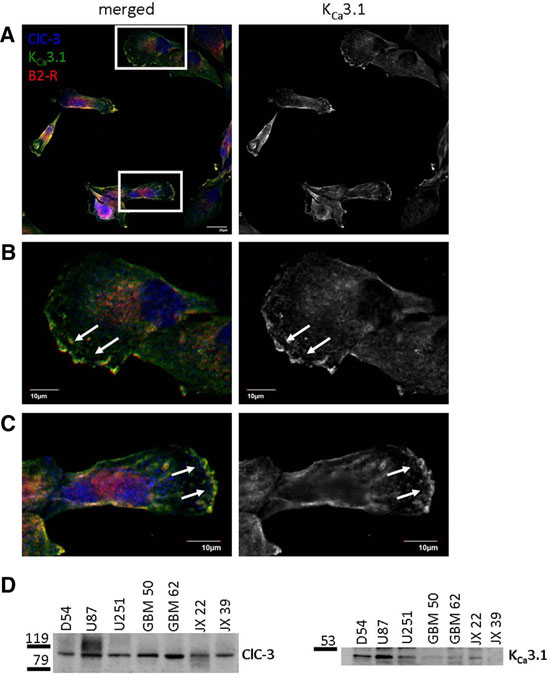
Colocalization and expression of KCa3.1, ClC-3 and B2R in glioma cells.A-C. Immunocytochemical staining of human D54 glioma cells using Mouse Anti-KCNN4 (KCa3.1, SK4) (extracellular) Antibody (#ALM-051). KCNN4, ClC-3 and B2R co-localize to the edges of cells. D. Western blot analysis of a number of glioma cells using Anti-CLC-3 (CLCN3) Antibody (#ACL-001) and Mouse Anti-KCNN4 (KCa3.1, SK4) (extracellular) Antibody.Adapted from Cuddapah, V.A. et al. (2013) with permission of the Society for Neuroscience.
Applications
Citations
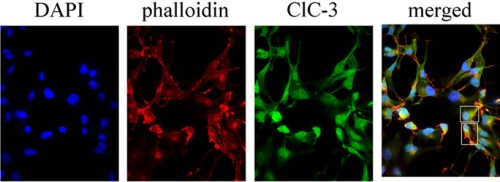
Expression of CLC-3 in human D54 glioma cells.
Immunocytochemical staining of human D4 glioma cells using Anti-CLC-3 (CLCN3) Antibody (#ACL-001), (green). CLC-3 co-localizes with cortical actin (stained with phalloidin, red) on the plasma membrane. DAPI (blue) is used to stain nuclei.
Adapted from Cuddapah, V.A. and Sontheimer, H. (2010) J. Biol. Chem. 285, 2010. with permission of the American Society for Biochemistry and Molecular Biology.
- Western blot analysis of rat A10 Vascular smooth muscle cell lysate. Tested in siRNA-treated cells.
Ma, M.M. et al. (2016) Br. J. Pharmacol. 173, 529. - Immunohistochemical staining and western blot analysis of mouse DRG sections and lysates respectively (1:200). Tested in CLC3-/- mice.
Pang, R.P. et al. (2016) Neuropharmacology 110, 181.
- Rat A10 Vascular smooth muscle cell lysate. Tested in siRNA-treated cells.
Ma, M.M. et al. (2016) Br. J. Pharmacol. 173, 529. - Mouse DRG lysate (1:500).
Pang, R.P. et al. (2016) Neuropharmacology 110, 181. - Human fibroblast lysate.
Lakoma, J. et al. (2016) J. Cell. Physiol. 231, 192. - Human mG-63 osteosarcoma cell lysate.
Du, S. and Yang, L. (2015) Int. Clin. Exp. Pathol. 8, 1622. - Mouse endothelial progenitor cells (1:500).
Liu, J. et al. (2013) Apoptosis 18, 1262. - tsA cells expressing CLC-3.
Farmer, L.M. et al. (2013) J. Physiol. 591, 1001. - Human glioma lysates (1:500).
Cuddapah, V.A. et al. (2013) J. Neurosci. 33, 1427.
- tsA cells expressing CLC-3.
Farmer, L.M. et al. (2013) J. Physiol. 591, 1001.
- Mouse DRG sections (1:200). Also tested in CLC3-/- mice.
Pang, R.P. et al. (2016) Neuropharmacology 110, 181. - Rat brain sections (1:100).
Farmer, L.M. et al. (2013) J. Physiol. 591, 1001.
- sA cells expressing CLC-3.
Farmer, L.M. et al. (2013) J. Physiol. 591, 1001. - Human D54 glioma cells.
Cuddapah, V.A. and Sontheimer, H. (2010) J. Biol. Chem. 285, 2010.
- Zhang, H. et al. (2013) Int. J. Biochem. Cell Biol. 45, 672.
- Cuddapah, V.A. et al. (2012) Am. J. Physiol. 302, C527.
- Ohtaki, H. et al. (2012) J. Neurosci. Res. 90, 2163.
- Wang, L. et al. (2012) Am. J. Physiol. 303, C14.
- Yang, L. et al. (2011) J. Cell. Physiol. 226, 2516.
- Liu, Y.J. et al. (2010) Hypertension 56, 445.
- Schenck, S. et al. (2009) Nat. Neurosci. 12, 156.
- Habela, C.W. et al. (2008) J. Neurosci. 28, 9205.
- Li, H. et al. (2008) Arch Otolaryngol Head Neck Surg. 134, 301.
- Yin, Z. et al. (2008) Am. J. Physiol. Cell Physiol. 294, C535.
- Kasinathan, R.S. et al. (2007) FEBS Lett. 581, 5407.
- Li, S.J. et al. (2006) J. Biochem. 139, 813.
- Lemonnier, L. et al. (2005) Endocr. Relat. Cancer 12, 335.
- Hara-Chikuma, M. et al. (2005) J. Biol. Chem. 280, 1241.
- Zhou, J-G. et al. (2005) J. Biol. Chem. 280, 7301.
- Fadool, D.A. et al. (2004) Neuron 41, 389.
- Lemonnier, L. et al. (2004) Cancer Res. 64, 4841.
- Robinson, N.C. et al. (2004) J. Physiol. 556.2, 353.
- Koni, P.A. et al. (2003) J. Biol. Chem. 278, 39443.
- Olsen, M.L. et al. (2003) J. Neurosci. 23, 5572.
- Hermoso, M. et al. (2002) J. Biol. Chem. 277, 40066.
- Tsuboi, T. et al. (2002) Biophys. J. 83, 172.
- Weng, T.X. et al. (2002) Am. J. Physiol. Cell Physiol. 283, 839.
- Duan, D. et al. (2001) J. Physiol. 531, 437.
- Britton, F.C. et al. (2000) Am. J. Physiol. 279, H2225.
- Weylandt, K-H. et al. (2001) J. Biol. Chem. 276, 17461.
- Wang, L. et al. (2000) J. Physiol. 524.1, 63.
- Wills, N.K. et al. (2000) Invest. Ophalmol. Vis. Sci. 41, 4247.
- Shimada, K. et al. (2000) Am J. Physiol. 279, G268.
- Luyckx, V.A. et al. (1999) Proc. Natl. Acad. Sci. USA 96, 12174.
