Overview
- Peptide (C)KDLARYRLGKGGLE, corresponding to amino acid residues 783-796 of rat CLC-7 (Accession P51799). Intracellular, C-terminal.
- Mouse kidney and rat brain lysates (1:200-1:1000).
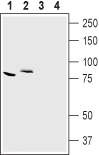 Western blot analysis of mouse kidney (lanes 1 and 3) and rat brain (lanes 2 and 4) lysates:1,2. Anti-CLC-7 (CLCN7) Antibody (#ACL-008), (1:200).
Western blot analysis of mouse kidney (lanes 1 and 3) and rat brain (lanes 2 and 4) lysates:1,2. Anti-CLC-7 (CLCN7) Antibody (#ACL-008), (1:200).
3,4. Anti-CLC-7 (CLCN7) Antibody, preincubated with CLC-7/CLCN7 Blocking Peptide (#BLP-CL008).
Chloride channels (CLCs) are a family of nine anion channels and anion/proton exchangers. While CLC-3 through CLC-7 are anion/proton exchangers, other members of the family seem to function only as anion channels. CLC exchangers, including CLC-7, are mainly expressed in intracellular compartments such as endosomes and lysosomes and are involved in housekeeping regulation of these organelles.
The CLC-7 protein is assembled as a dimer with each subunit consisting of 18 transmembrane helices followed by a cytosolic carboxy-terminus that contains two conserved cystathionine-β-synthase (CBS) domains. Three separate chloride binding sites within each subunit were identified in three-dimensional structures, Sint, Scen, and Sext, named after their position relative to the extra- or intra-cellular side of the plasma membrane. The central position forms a significant part of the selectivity filter with the conserved amino acids S107, I109, F357, and Y445 in addition to the less well conserved F348 and I356 coordinating the chloride ion within the conduction pathway1.
CLC-7 is vital for the proper function of osteoclast cells. The neutralizing current through CLC-7 is required for an efficient acidification of the cell’s resorption lacuna. CLC-7 can be inserted into this specialized plasma membrane domain by an exocytotic insertion from late endosomal–lysosomal membranes. Mice deficient of CLC-7 protein show severe osteoporosis and retinal degeneration2.
