Overview
- Peptide (C)KSEKEQFLLKDTER, corresponding to amino acid residues 245-258 of human CRACR2A (Accession Q9BSW2). Intracellular.
- Human acute T cell leukemia (Jurkat) and rat stomach lysates (1:400-1:2000).
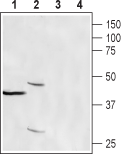 Western blot analysis of human T cell leukemia (Jurkat) (lanes 1 and 3) and rat stomach (lanes 2 and 4) lysates:1,2. Anti-CRACR2A (EFCAB4B) Antibody (#ACC-324), (1:400).
Western blot analysis of human T cell leukemia (Jurkat) (lanes 1 and 3) and rat stomach (lanes 2 and 4) lysates:1,2. Anti-CRACR2A (EFCAB4B) Antibody (#ACC-324), (1:400).
3,4. Anti-CRACR2A (EFCAB4B) Antibody, preincubated with CRACR2A/EFCAB4B Blocking Peptide (#BLP-CC324).
Calcium is a vital ion for the functioning of many intracellular processes. When Ca2+ stored in the endoplasmic reticulum is depleted, there is an activation of store-operated channels that results in the influx of Ca2+ from outside the cell. In non-excitable cells and especially in immune cells, most of this influx is carried out by calcium release activated calcium channels also known as “CRACs”1.
Mediation of signals from the ER to CRACs and activation of the CRAC channel is conducted by two modulators that physically cluster with the CRAC channel known as Orai1 and STIM1. STIM1 is a single transmembrane segment protein that has a long C-terminal cytoplasmic region and an N-terminus which detects ER Ca2+ depletion. Orai1 is a macromolecular complex with linkers of varying lengths between the ER and plasma membranes.
Novel findings suggest the existence of a complex comprised of Orai1, STIM1 and additional proteins. One of these proteins is an EF-hand protein named CRACR2A which regulates Orai-STIM interaction. An EF-hand is a structural domain found in Ca2+ binding proteins consisting of two alpha helices linked by a short loop region.
CRACR2A interacts with the cytoplasmic regions of both Orai1 and STIM1 thus creating a tertiary complex. Under high concentrations of Ca2+, CRACR2A caused a dissociation of Orai1 and STIM1 and a mutated CRACR2A was found, in one study, to cause elevated Ca2+ levels that caused cell death in T cells2.
Further research is required to fully determine the activation and regulation mechanisms of CRACs and this can be performed by better understanding CRACR2A and its binding proteins.
