Overview
- Peptide (C)YGVKESRKRREAGS, corresponding to amino acid residues 129-142 of rat ENaCγ (Accession P37091). Extracellular.
- Rat lung lysate (1:200).
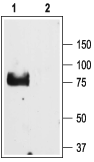 Western blot analysis of rat lung lysate:1. Anti-ENaC γ (SCNN1G) (extracellular) Antibody (#ASC-011), (1:200).
Western blot analysis of rat lung lysate:1. Anti-ENaC γ (SCNN1G) (extracellular) Antibody (#ASC-011), (1:200).
2. Anti-ENaC γ (SCNN1G) (extracellular) Antibody, preincubated with ENaC gamma/SCNN1G (extracellular) Blocking Peptide (#BLP-SC011).
- Guinea pig inner ear sections (1:300) (Mori, Y. et al. (2009) J. Physiol. Sci. 59, 355.).
The amiloride-sensitive epithelial Na+ channel (ENaCs) family includes 4 members: ENaCα, β, γ and δ. The ENaC channel is located in the luminal (apical) plasma membrane of several epithelial tissues such as kidney, lung, salivary glands and skin.
The functional channel is believed to be a multimer including a α (or δ) and a β and γ subunits with a likely stoichiometry of α2βγ.
ENaC channel enable entry of Na+ into the cell along its electrochemical gradient and thus has a central role in the maintenance of renal Na+ balance (and hence blood pressure) and liquid balance in the lung. Indeed, genetic mutations in the ENaC subunits causes Liddle’s syndrome (a form of hypertension) or pseudohypoaldosteronism type 1 (PHA) that is characterized by hypotension.
The ENaC channel is voltage-independent and is constitutively active in epithelia although it is modulated by several different mechanisms. One of the main mechanisms is the controlled internalization of the channel that is dependent on the β or γ subunits. Indeed, mutations in the C-termini of these subunits reduce endocytosis of the channel leading to the accumulation of ENaC in the cell membrane and causing a phenotype that is consistent with that of Liddle’s syndrome.
