Overview
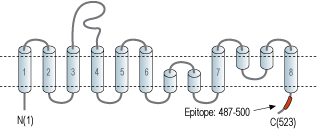
- Peptide (C)EPTILDNEDSDTKK, corresponding to amino acid residues 487-500 of rat EAAT3 (Accession P51907). Intracellular, C-terminus.
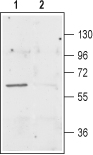 Western blot analysis of rat brain membranes:1. Anti-EAAT3 (EAAC1) Antibody (#AGC-023), (1:400).
Western blot analysis of rat brain membranes:1. Anti-EAAT3 (EAAC1) Antibody (#AGC-023), (1:400).
2. Anti-EAAT3 (EAAC1) Antibody, preincubated with EAAT3/EAAC1 Blocking Peptide (#BLP-GC023). Western blot analysis of mouse kidney lysate:1. Anti-EAAT3 (EAAC1) Antibody (#AGC-023), (1:400).
Western blot analysis of mouse kidney lysate:1. Anti-EAAT3 (EAAC1) Antibody (#AGC-023), (1:400).
2. Anti-EAAT3 (EAAC1) Antibody, preincubated with EAAT3/EAAC1 Blocking Peptide (#BLP-GC023).
 Expression of EAAT3 in rat cerebellumImmunohistochemical staining of perfusion-fixed free-floating frozen rat brain sections with Anti-EAAT3 (EAAC1) Antibody (#AGC-023). A. EAAT3 staining (green) appears in the molecular layer (Mol) and around the soma of Purkinje cells (arrow). B. Parvalbumin (red), a marker of Purkinje and interneuronal cells, stains the same section. C. Merge of the images demonstrates expression of EAAT3 around the soma of Purkinje cells. DAPI is used as the counterstain.
Expression of EAAT3 in rat cerebellumImmunohistochemical staining of perfusion-fixed free-floating frozen rat brain sections with Anti-EAAT3 (EAAC1) Antibody (#AGC-023). A. EAAT3 staining (green) appears in the molecular layer (Mol) and around the soma of Purkinje cells (arrow). B. Parvalbumin (red), a marker of Purkinje and interneuronal cells, stains the same section. C. Merge of the images demonstrates expression of EAAT3 around the soma of Purkinje cells. DAPI is used as the counterstain.
- Mouse astrocytes and cerebellar neurons (Guitart, K. et al. (2015) J. Neurochem. 133, 558).
- Tzingounis, A.V. and Wadiche, J.I. (2007) Nat. Rev. Neurosci. 8, 935.
- Beart, P.M. and O’Shea, R.D. (2007) Br. J. Pharmacol. 150, 5.
L-Glutamate (Glu) is an abundant amino acid that functions as the major excitatory neurotransmitter in the central nervous system. However, excess of Glu in the extracellular synaptic milieu leads to neuronal cell death by a process known as excitotoxicity.
The extracellular levels of Glu are regulated by a family of high affinity plasma membrane transporters called excitatory amino acid transporters (EAATs) which are responsible for the re-uptake of Glu into the cells1,2.
The EAAT family includes five members (EAAT1-EAAT5) that are members of the solute carrier family 1 (SLC1) of Na+-dependent transporters that also includes the neutral amino acid transporters ASCT1 and ASCT2.
The Glu transporters present an unusual topology of eight transmembrane domains with two re-entrant loops and intracellular N- and C- termini. The transporter is likely assembled as a trimer where each monomer is a functional unit capable of binding the Glu substrate.
The transport of Glu into the cells by the EAAT transporters is coupled to the Na+ and K+ electrochemical gradient as a driving force. Hence, the uptake of Glu is dependent on the co-transport of three Na+ and one H+ ions, and the counter transport of one K+ ion.
In addition, to the well documented Glu uptake, the EAAT transporters show a Glu-independent Cl- conductance. The physiological significance of the Cl- current through the EAATs is currently unknown1,2.
EAAT2 as well as EAAT1 are expressed predominantly in glia cells, while EAAT3 (Excitatory Amino Acid Transporter 3), EAAT4 and EAAT5 are mostly expressed in neurons.
As mentioned, EAAT transporters represent the only (significant) mechanism for removal of glutamate from the extracellular fluid and hence are essential for the long-term maintenance of low and non-toxic concentrations of glutamate and the preservation of normal excitatory synaptic transmission.
In addition to Glu uptake, the glutamate transporters provide glutamate for the synthesis of g-Aminobutyric acid (GABA), glutathione and protein, suggesting an interactive role between EAATs and cellular metabolism1,2.
Dysregulation of EAAT activities has been implicated in several neurodegenerative disorders such as Alzheimer’s disease, traumatic brain injury, epilepsy and schizophrenia, suggesting that EAATs can be a useful target for the treatment of these conditions1,2.
Application key:
Species reactivity key:
Anti-EAAT3 (EAAC1) Antibody (#AGC-023) is a highly specific antibody directed against an epitope of the rat Excitatory amino acid transporter 3. The antibody can be used in western blot, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize EAAT3 from rat, mouse, and human samples.
Applications
Citations
- Human Schwann cell lysate.
Rohrbeck, A. et al. (2015) Neurochem. Int. 90, 232.
- Mouse astrocytes and cerebellar neurons.
Guitart, K. et al. (2015) J. Neurochem. 133, 558
