Overview
- Peptide (C)KFVYKEEHPFEKRR, corresponding to amino acid residues 2-15 of rat GABARAP (Accession P60517). Intracellular, N-terminus.

GABA receptor associated protein-like 1 (11/14 amino acid residues identical), (Accession Q0VGK0).
GABA receptor associated protein-like 3 (11/14 amino acid residues identical), (Accession Q9BY60).
May recognize GABA receptor associated protein-like 2 (8/14 amino acid residues identical), (Accession P60522).
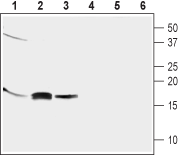 Western blot analysis of mouse brain (lanes 1 and 4), rat brain (lanes 2 and 5) and human neuroblastoma (SH-SY5Y) cell line (lanes 3 and 6) lysates:1-3. Anti-GABARAP Antibody (#AIP-016), (1:800).
Western blot analysis of mouse brain (lanes 1 and 4), rat brain (lanes 2 and 5) and human neuroblastoma (SH-SY5Y) cell line (lanes 3 and 6) lysates:1-3. Anti-GABARAP Antibody (#AIP-016), (1:800).
4-6. Anti-GABARAP Antibody, preincubated with GABARAP Blocking Peptide (#BLP-IP016).
 Expression of GABARAP in rat cortex.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Anti-GABARAP Antibody (#AIP-016), (1:1000), followed by donkey anti-rabbit-biotin and Streptavidin-Cy3. A. GABARAP immunoreactivity (red) appears in cortical neurons (arrows). B. Pre-incubation of the antibody with GABARAP Blocking Peptide (#BLP-IP016), suppressed staining. Cell nuclei are stained with DAPI (blue).
Expression of GABARAP in rat cortex.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Anti-GABARAP Antibody (#AIP-016), (1:1000), followed by donkey anti-rabbit-biotin and Streptavidin-Cy3. A. GABARAP immunoreactivity (red) appears in cortical neurons (arrows). B. Pre-incubation of the antibody with GABARAP Blocking Peptide (#BLP-IP016), suppressed staining. Cell nuclei are stained with DAPI (blue).
- Wang, H. et al. (1999) Nature 397, 69.
- Chen, Z. and Olsen, R.W. (2007) J. Neurochem. 100, 279.
GABA(A) receptors are ligand-gated chloride channels that mediate inhibitory neurotransmission. GABA associated protein (GABARAP) family interacts specifically with the γ1, γ2S and γ2L subunits of the GABA(A) receptor. GABARAP is expressed in a variety of tissues including heart, brain, placenta, liver and others. The genetic sequence encoding GABARAP is similar to microtubule-associated proteins 1A and 1B1.
GABARAP has an asymmetric structure with a basic N-terminal region and an acidic C terminus. GABARAP has a compact fold consisting of four or five-stranded β-sheets with two α-helices on each side. The outer strands of the β-sheets are aligned anti-parallel to the inner strands and helices α3 and α4 are located on one side of the sheet. The presence of α1 and α2 is a unique feature of GABARAP and forms a hydrophilic patch suitable for protein-protein interface. GABARAP exists in a monomeric conformation at low protein concentration and a head-to-tail oligomeric conformation in high salt conditions.
GABARAP is involved in intracellular trafficking and is an integral part of the intra-Golgi transport machinery. Thus it is detected in cellular compartments such as the Golgi apparatus and endoplasmic reticulum. GABARAP affects GABA(A) receptors’ cell surface expression by increasing their intracellular trafficking. Disruption of cellular microtubules prevents the clustering of both GABARAP and GABA(A) receptors. GABARAP also interacts with other receptors such as the glycine receptor at the postsynaptic site2.
Application key:
Species reactivity key:
Anti-GABARAP Antibody (#AIP-016) is a highly specific antibody directed against an epitope of the rat GABA receptor associated protein. The antibody can be used in western blot analysis. It has been designed to recognize GABARAP from human, mouse and rat samples. Due to high homology with GABA receptor associated protein-like proteins the antibody will recognize GABA receptor associated protein-like 1 and 3 and may recognize GABA receptor associated protein-like 2.
