Overview
- Peptide (C)DGNDVEFSWLRGND, corresponding to amino acid residues 184-197 of rat GABA(A) π Receptor (Accession O09028). Extracellular, N-terminus.

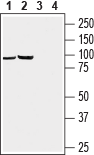 Western blot analysis of rat brain (lanes 1 and 3) and mouse brain (lanes 2 and 4) lysates:1, 2. Anti-GABA(A) π Receptor/GABRP (extracellular) Antibody (#AGA-019), (1:200).
Western blot analysis of rat brain (lanes 1 and 3) and mouse brain (lanes 2 and 4) lysates:1, 2. Anti-GABA(A) π Receptor/GABRP (extracellular) Antibody (#AGA-019), (1:200).
3, 4. Anti-GABA(A) π Receptor/GABRP (extracellular) Antibody, preincubated with GABA(A) π Receptor (extracellular) Blocking Peptide (#BLP-GA019).
 Expression of GABA(A) π Receptor in mouse hippocampus.Immunohistochemical staining of perfusion-fixed frozen mouse brain sections with Anti-GABA(A) π Receptor/GABRP (extracellular) Antibody (#AGA-019), (1:200), followed by goat anti-rabbit-AlexaFluor-488. A. Staining in the mouse hippocampal dentate gyrus (DG) region showed GABARP immunoreactivity (green) in hilar (H) neurons (vertical arrows). B. Pre-incubation of the antibody with GABA(A) π Receptor/GABRP (extracellular) Blocking Peptide (BLP-GA019), suppressed staining. Cell nuclei are stained with DAPI (blue).
Expression of GABA(A) π Receptor in mouse hippocampus.Immunohistochemical staining of perfusion-fixed frozen mouse brain sections with Anti-GABA(A) π Receptor/GABRP (extracellular) Antibody (#AGA-019), (1:200), followed by goat anti-rabbit-AlexaFluor-488. A. Staining in the mouse hippocampal dentate gyrus (DG) region showed GABARP immunoreactivity (green) in hilar (H) neurons (vertical arrows). B. Pre-incubation of the antibody with GABA(A) π Receptor/GABRP (extracellular) Blocking Peptide (BLP-GA019), suppressed staining. Cell nuclei are stained with DAPI (blue).
- Akabas, M.H. (2004) Int. Rev. Neurobiol. 62, 1.
- Chua, H.C. and Chebib, M. (2017) Adv. Pharmacol. 79, 1.
- Chen, Z.W. et al. (2019) PloS Biol. 17, e3000157.
- Olsen, R.W. (2018) Neuropharmacology 136, 10.
The GABA(A) receptor (GABAAR) is a pentameric transmembrane protein, member of the Cys-loop family of neurotransmitter-gated ion channels. Its ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Binding of GABA induces the opening of an anion-selective ion channel that is an integral part of the receptor protein. The opening of this channel enables the entrance or exit of Cl- ions according to the resting potential of the cell surroundings. This causes an inhibitory effect on neurotransmission by decreasing the chance of a successful action potential transmission1. GABAARs occur in all organisms that have a nervous system, and they are mostly located in neuronal tissues. Due to their wide distribution within the nervous system and their basic function, they play a role in virtually all brain functions.
These pentameric receptors occur with diverse subunit composition, at least 19 GABAAR subunits are identified in the human genome and the heteromeric nature of GABAAR allows for an enormous range of theoretically possible subunit combinations. Yet, experimental evidence suggests that only a few dozen combinations exist in vivo2.
Members of the cys-loop family of ion channels share a similar structure that consists of an extracellular N- and C-terminal domains, four helical membrane-spanning segments (M1, M2, M3, and M4) and a 15-residue disulfide-linked loop in the extracellular domain3.
The pivotal role of GABAARs in all brain functions makes them a desirable drug target and an impressive range of clinically used therapeutics are known to bind to distinct sites found on GABAARs to modulate receptor function4.
Application key:
Species reactivity key:
Anti-GABA(A) π Receptor/GABRP (extracellular) Antibody (#AGA-019) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot analysis. It has been designed to recognize GABRP from human, rat, and mouse samples.
