Overview
- Peptide (C)RHHKSPMLNLFQD, corresponding to amino acid residues 350-362 of rat GlyRα1 (Accession P07727). 2nd intracellular loop.

 Western blot analysis of rat DRG (lanes 1 and 4), mouse brain (lanes 2 and 5) and rat brain (lanes 3 and 6) lysates:1-3. Anti-Glycine Receptor α1 Antibody (#AGR-001), (1:400).
Western blot analysis of rat DRG (lanes 1 and 4), mouse brain (lanes 2 and 5) and rat brain (lanes 3 and 6) lysates:1-3. Anti-Glycine Receptor α1 Antibody (#AGR-001), (1:400).
4-6. Anti-Glycine Receptor α1 Antibody, preincubated with Glycine Receptor α1 Blocking Peptide (#BLP-GR001).
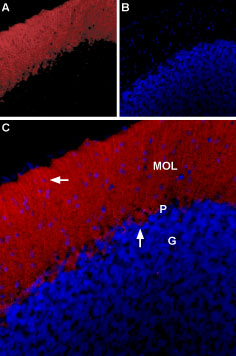 Expression of GlyR α1 in rat cerebellumImmunohistochemical staining of rat cerebellum using Anti-Glycine receptor α1 Antibody (#AGR-001), (1:100). A. GlyRα1 staining (red) is revealed in the molecular layer (MOL), Bergmann glia (horizontal arrow) and in Purkinje cells (a vertical arrow). B. Nuclear staining using DAPI as the counterstain (blue). C. Merged images of A and B.
Expression of GlyR α1 in rat cerebellumImmunohistochemical staining of rat cerebellum using Anti-Glycine receptor α1 Antibody (#AGR-001), (1:100). A. GlyRα1 staining (red) is revealed in the molecular layer (MOL), Bergmann glia (horizontal arrow) and in Purkinje cells (a vertical arrow). B. Nuclear staining using DAPI as the counterstain (blue). C. Merged images of A and B.
- Werman, R. et al. (1967) Nature 214, 681.
- Betz, H. et al. (1992) Q. Rev. Biophys. 25, 381.
- Kuhse, J. et al. (1990) J. Biol. Chem. 265, 223.
- Malosio, M.L. et al. (1991) Embo J. 10, 2401.
- Durisic, N. et al. (2012) J. Neurosci. 32, 12915.
- Bakker, M.J. et al. (2006) Lancet Neurol. 5, 513.
Glycine receptors (GlyRs) mediate ionotropic inhibitory neurotransmission in the CNS where they play an essential role in inhibition of motor neurons in the spinal cord and brainstem1. The glycine receptor is composed of three ligand-binding subunits of 48 kDa (α) and two homologous polypeptides of 58 kDa (β) that span the postsynaptic membrane in a pentameric arrangement to form a Cl--selective channel2. So far, at least three different α-subunit variants of the GlyR have been identified: α1, α2 and α33. Homo-oligomeric α2 receptors have a low strychnine-binding affinity and are considered to represent a neonatal form of GlyR, which is replaced during development by receptors containing the α1 subunit. Adult GlyRs are predominantly composed of α1 and β subunits, the α3 subunit being expressed at low levels in only a few brain regions4.
GlyRα1 subunits are widely expressed and contribute to many processes in the CNS, including inflammatory pain perception, modulation of auditory and visual pathways, and neurotransmission in the cerebellar cortex5.
Mutations in glycine receptor α1 subunits cause human hereditary hyperekplexia, a disorder characterized by an exaggerated startle response, which is a consequence of the role of GlyRs in motor reflex circuits of the spinal cord6.
Application key:
Species reactivity key:
Alomone Labs is pleased to offer a highly specific antibody directed against an epitope of the rat glycine receptor α1. Anti-Glycine Receptor α1 Antibody (#AGR-001) can be used in western blot and immunohistochemical staining. It has been designed to recognize GlyRα1 from rat, mouse and human samples.
