Overview
- Peptide CVIGDHPKIQIKNS, corresponding to amino acid residues 333-346 of rat GlyT2 (Accession P58295). 2nd extracellular loop.

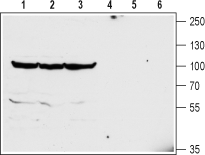 Western blot analysis of mouse brain (lanes 1 and 4), rat brain (lanes 2 and 5) and rat cerebellum (lanes 3 and 6) lysates:1-3. Anti-GlyT2 (SLC6A5) (extracellular) Antibody (#AGT-012), (1:200).
Western blot analysis of mouse brain (lanes 1 and 4), rat brain (lanes 2 and 5) and rat cerebellum (lanes 3 and 6) lysates:1-3. Anti-GlyT2 (SLC6A5) (extracellular) Antibody (#AGT-012), (1:200).
4-6. Anti-GlyT2 (SLC6A5) (extracellular) Antibody, preincubated with GlyT2/SLC6A5 (extracellular) Blocking Peptide (#BLP-GT012).
 Expression of glycine transporter 2 in mouse brain stemImmunohistochemical staining of immersion-fixed, free floating mouse brain frozen sections using Anti-GlyT2 (SLC6A5) (extracellular) Antibody (#AGT-012), (1:200). A. GlyT2 (red) is expressed in neurons (arrow). B. Dapi staining of cell nuclei (blue) is used as a general cellular marker.
Expression of glycine transporter 2 in mouse brain stemImmunohistochemical staining of immersion-fixed, free floating mouse brain frozen sections using Anti-GlyT2 (SLC6A5) (extracellular) Antibody (#AGT-012), (1:200). A. GlyT2 (red) is expressed in neurons (arrow). B. Dapi staining of cell nuclei (blue) is used as a general cellular marker.
- Zafra, F. et al. (2008) IUBMB Life. 60, 810.
- Chen, N. et al. (2004) Pflugers. Arch. 447, 519.
- Supplisson, S. et al. (2002) FEBS Lett. 529, 93.
- Kristensen, A.S. et al. (2011) Pharmacol. Rev. 63, 585.
- Olivares, L. et al. (1995) J. Biol. Chem. 270, 9437.
- Massari, S. et al. (2005) J. Biol. Chem. 280, 7388.
- Liu, Q.R. et al. (1993) J. Biol. Chem. 268, 22802.
- Rees, M.I. et al. (2006) Nat. Genet. 38, 801.
Apart from its obvious biochemical functions, glycine is also an important inhibitory neurotransmitter. Following depolarization, glycine is released from synaptic vesicles, binds to glycine receptors (GlyRs) on postsynaptic membranes thereby causing hyperpolarization of postsynaptic neurons due to the massive influx of Cl- ions. Glycine is then taken up from the synaptic cleft via the glycine transporters GlyT1 and GlyT21.
GlyT1 and GlyT2 belong to the SLC6, Na+/Cl- dependent transporter family, of which members include transporters for GABA, serotonin, dopamine and norepinephrine2. Like all SLC6 members, GlyT1 and GlyT2 have 12 transmembrane domains and intracellular N- and C-terminals. Both can be found in different splice variants3,4. SLC6 transporters undergo post-translational modifications. For instance, GlyT1 and GlyT2 are glycosylated, which is important for their membrane trafficking5. Phosphorylation of these two transporters also takes place in a PKC-dependent manner, which may lead to down regulation of both transporters6. Pharmacologically, GlyT1 and GlyT2 can be differentiated by applying sarcosine which inhibits GlyT1 but not GlyT27.
GlyT1 and GlyT2 are broadly expressed in the nervous system; GlyT1 is concentrated in glial cells, while GlyT2 is present in glycenergic neurons in the spinal cord, brainstem and cerebellum1. GlyT1 can also be detected in the pancreas, uterus, stomach, spleen, liver and retina4.
GlyT1 has become a target for the treatment of schizophrenia, although a defect of the protein is not directly associated with the disorder. Inhibiting GlyT1 should lead to the increase in glutamatergic pathways, thereby decreasing psychotic effects in schizophrenic individuals. GlyT2 has been associated with hyperekplexia, a motor disorder characterized by neonatal hypertonia and startle reflex8.
Application key:
Species reactivity key:
Alomone Labs is pleased to offer a highly specific antibody directed against an extracellular epitope of the rat glycine transporter 2. Anti-GlyT2 (SLC6A5) (extracellular) Antibody (#AGT-012) can be used in western blot and immunohistochemistry analysis and has been designed to recognize GlyT2 from rat, mouse and human samples.
