Overview
- Peptide (C)KPNSASNSRDDGNSVYPSK, corresponding to amino acid residues 6-24 of rat HCN1 (Accession Q9JKB0). Intracellular, N-terminus.

 Western blot analysis of rat brain membrane:1. Anti-HCN1 Antibody (#APC-056), (1:200).
Western blot analysis of rat brain membrane:1. Anti-HCN1 Antibody (#APC-056), (1:200).
2. Anti-HCN1 Antibody, preincubated with HCN1 Blocking Peptide (#BLP-PC056).- Human HCN1 transfected in HEK-293 cells (Stieber, J. et al. (2005) J. Biol. Chem. 280, 34635.).
 Expression of HCN1 in mouse cerebellumImmunohistochemical staining mouse cerebellum using Anti-HCN1 Antibody (#APC-056). A. HCN1 (green) appears in the cerebellar pinceau. B. Calcium binding calbindinD28-K (red), a marker of Purkinje neurons, is stained in the same section. C. Merge of the images demonstrates the position of the HCN1-positive pinceau structures at the axon initial segment of Purkinje neurons.
Expression of HCN1 in mouse cerebellumImmunohistochemical staining mouse cerebellum using Anti-HCN1 Antibody (#APC-056). A. HCN1 (green) appears in the cerebellar pinceau. B. Calcium binding calbindinD28-K (red), a marker of Purkinje neurons, is stained in the same section. C. Merge of the images demonstrates the position of the HCN1-positive pinceau structures at the axon initial segment of Purkinje neurons.
 Expression of HCN1 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized rat DRG primary culture. Cells were stained with Anti-HCN1 Antibody (#APC-056), (1:300), followed by goat anti-rabbit-AlexaFluor-488 secondary antibody (green). Nuclear staining of cells using the cell-permeable dye Hoechst 33342 (blue).
Expression of HCN1 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized rat DRG primary culture. Cells were stained with Anti-HCN1 Antibody (#APC-056), (1:300), followed by goat anti-rabbit-AlexaFluor-488 secondary antibody (green). Nuclear staining of cells using the cell-permeable dye Hoechst 33342 (blue).
- Much, B. et al. (2003) J. Biol. Chem. 278, 43781.
- Notomi, T. and Shigemoto R. (2004) J. Comp. Neurol. 471, 241.
- Gravante, B. et al. (2004) J. Biol. Chem. 279, 43847.
Hyperpolarization-activated cation currents (Ih) appear in the heart and the brain and have a crucial role in controlling electrical pacemaker activity, contributing to biological processes such as heartbeat, sleep-wake cycle and synaptic plasticity.1,2
The Ih currents are generated by the hyperpolarization-activated cyclic nucleotide-gated channel family (HCN), which is comprised of four homologous members, HCN1-4.
Each HCN subunit consists of six transmembrane domains (TM), a pore region between TM5-TM6 and a binding domain for cyclic nucleotides (CNBD) in the cytoplasmic C-terminus.2 The HCN subunits can form functional homomers and can also co-assemble into functional heteromers.2
The channels are closely related to each other and share a homology of about 60%. However, their similarity decreases in the cytoplasmic N- and C-termini. The channels HCN1-4 mainly differ from each other in their speed of activation and the extent to which they are modulated by cAMP. HCN1, weakly affected by cAMP, is the fastest channel, followed by HCN2, HCN3 and HCN4.
HCN1 is extensively expressed in the brain, in specific areas like the neocortex, hippocampus, cerebellum and superior colliculus.2,3
Application key:
Species reactivity key:
Anti-HCN1 Antibody (#APC-056) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunohistochemistry and immunocytochemistry applications. It has been designed to recognize HCN1 from human, rat, and mouse samples.
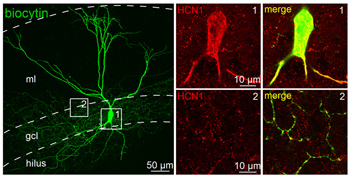 Expression of HCN1 in perisoma-inhibiting interneurons (PIIs) of the dentate gyrus.Immunocytochemical staining of perisoma-inhibiting interneurons using Anti-HCN1 Antibody (#APC-056). HCN1 expression (green) is detected in the soma and axon colaterals. Biocytin was used to label neurons.Adapted from Elgueta, C. et al. (2015) with permission of the Society for Neuroscience.
Expression of HCN1 in perisoma-inhibiting interneurons (PIIs) of the dentate gyrus.Immunocytochemical staining of perisoma-inhibiting interneurons using Anti-HCN1 Antibody (#APC-056). HCN1 expression (green) is detected in the soma and axon colaterals. Biocytin was used to label neurons.Adapted from Elgueta, C. et al. (2015) with permission of the Society for Neuroscience.Applications
Citations
- Western blot analysis of rat brain lysate. Tested in HCN1-/- rats.
Nishitani, A. et al. (2019) Brain Res. 1706, 209. - Immunohistochemistry of mouse heart sections. Tested in HCN1-/- mice.
Herrmann, S. et al. (2011) J. Mol. Cell. Cardiol. 51, 997.
- Rat brain lysate. Also tested in HCN1-/- rats.
Nishitani, A. et al. (2019) Brain Res. 1706, 209. - Rat synaptosomes.
Zhang, K. et al. (2016) Sci. Signal. 9, ra123. - Human HCN1 transfected in HEK-293 cells.
Stieber, J. et al. (2005) J. Biol. Chem. 280, 34635.
- Rat retina sections (1:50).
Li, Q. et al. (2017) Brain Struct. Funct. 222, 813. - Mouse brain sections.
Matsumoto-Makidono, Y. et al. (2016) Cell Rep. 16, 994. - Rat diaphragm sections (1:25).
Negrini, D. et al. (2016) Am. J. Physiol. 311, H892. - Mouse heart sections (1:100).
Fenske, S. et al. (2013) Circulation 128, 2585. - Mouse heart sections. Also tested in HCN1-/- mice.
Herrmann, S. et al. (2011) J. Mol. Cell. Cardiol. 51, 997.
- Hughes, D.I. et al. (2012) J. Physiol. 590, 3927.
- Klueva, J. et al. (2012) Neurosignals 20, 35.
- Liu, J. et al. (2012) Neurosci. Lett. 515, 168.
- Liu, C.Y. et al. (2012) J. Neurophysiol. 108, 1318.
- Ramakrishnan, N.A. et al. (2012) J. Biol. Chem. 287, 37628.
- Weng, X. et al. (2012) Pain 153, 900.
- Neitz, A. et al. (2011) Eur. J. Neurosci. 33, 1611.
