Overview
- Peptide (C)GGTPEERLKVAIT, corresponding to amino acid residues 184-196 of human FPR2/ALX (Accession P25090). 2nd extracellular loop.
- Human promyelocytic leukemia (HL-60) and chronic myelogenous leukemia (K562) cell lysates (1:200).
 Western blot analysis of Human HL-60 promyelocytic leukemia (lanes 1 and 3) and K562 chronic myelogenous leukemia (lanes 2 and 4) cell lysates:1,2. Anti-Human FPR2/ALX (extracellular) Antibody (#AFR-002), (1:200).
Western blot analysis of Human HL-60 promyelocytic leukemia (lanes 1 and 3) and K562 chronic myelogenous leukemia (lanes 2 and 4) cell lysates:1,2. Anti-Human FPR2/ALX (extracellular) Antibody (#AFR-002), (1:200).
3,4. Anti-Human FPR2/ALX (extracellular) Antibody, preincubated with Human FPR2/ALX (extracellular) Blocking Peptide (#BLP-FR002).
- Human minor salivary glands (1:200) (Nelson, J.W. et al. (2014) Am. J. Physiol. 306, C178.).
- Live intact chronic myelogenous leukemia (K562) cells (1:20).
- The blocking peptide is not suitable for this application.
Chemotactic factors from both Gram-positive and Gram-negative bacteria are short peptides with N-formyl methionine at the N-terminus (extensively reviewed in reference 1). These peptides are released from bacteria during infection and activate formyl peptide receptors (FPR), members of the G-protein coupled receptor (GPCR) superfamily. In humans, the FPR family consists mainly of three receptors, FPR1, FPR2/ALX (formerly FPRL1), and FPR3 (formerly FPRL2) which all couple to the Gi subtype of G-proteins and ultimately lead to the activation of phospholipase C and intracellular Ca2+ increase1,2.
FPRL1, or FPR2/ALX as it is commonly called, is a seven transmembrane protein like all GPCRs. This receptor was originally cloned by screening a HL60 neutrophil cDNA library with a FPR1 cDNA probe3. FPR2/ALX shares 69% identity with FPR1 and despite its high homology, it displays relatively low affinity for fmlf, the most potent N-formyl peptide released by bacteria3.
FPR1 was originally found in neutrophils and later found to be distributed in myeloid and non-myeloid cells as is the case for FPR2/ALX and FPR3 (FPR3 though is not expressed in neutrophils). FPR1 is also expressed in multiple organs and tissues including epithelial cells in organs with secretory functions, endocrine cells, liver hepathocytes, smooth muscle cells and endothelial cells, brain spinal cord and both motor and sensory neurons4. FPR2/ALX has a similar tissue distribution to that of FPR1.
While N-formyl peptides were the first peptides found to activate these receptors, the ligand diversity for FPR has proven to be quite broad and demonstrates to be both pro- and anti-inflammatory. They include peptidic ligands originating from bacterial and viral sources (including HIV), endogenous ligands such as chemokines and annexins, short peptides associated with inflammation and infection. Interestingly, β-amyloid peptide, a known marker for Alzheimer’s disease activates FPR2/ALX thereby directly linking these receptors to the neurodegenerative disease. Lipoxin A4 (LXA4) is the first identified endogenous ligand for FPR2/ALX and is an eicosanoid with potent anti-inflammatory characteristics1.
Considering that FPR2/ALX can be activated by two different types of ligands, this receptor can be categorized in two different GPCR subgroups: Formyl peptide receptors and Leukotriene receptors.
Application key:
Species reactivity key:
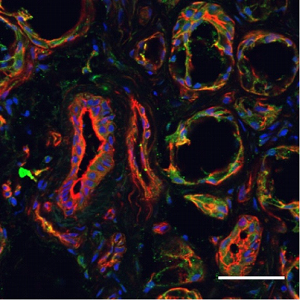 Expression of FPR2/ALX in human minor salivary glands.Immunohistochemical staining of human minor salivary gland (hMSG) sections using Anti-Human FPR2/ALX (extracellular) Antibody (#AFR-002). FPR2/ALX staining (green) is localized on the apical side of acinar and ductal hMSG. Phalloidin (red) is used to stain actin filaments and prodium iodide is used to stain nuclei. Adapted from Nelson, J.W. et al. (2014) Am. J. Physiol. 306, C178. with permission of The American Physiological Society.
Expression of FPR2/ALX in human minor salivary glands.Immunohistochemical staining of human minor salivary gland (hMSG) sections using Anti-Human FPR2/ALX (extracellular) Antibody (#AFR-002). FPR2/ALX staining (green) is localized on the apical side of acinar and ductal hMSG. Phalloidin (red) is used to stain actin filaments and prodium iodide is used to stain nuclei. Adapted from Nelson, J.W. et al. (2014) Am. J. Physiol. 306, C178. with permission of The American Physiological Society.
